DSM files patent for ‘snail fever’ fighting omega-6
The invention was comprised of polyunsaturated fatty acid arachidonic acid (ARA) and the only current treatment of the condition, the anthelmintic drug praziquantel.
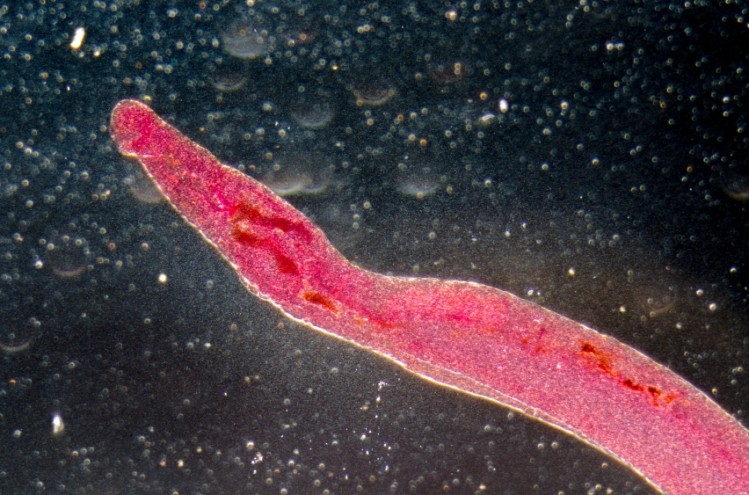
Schistosomiais is a condition caused by Schistosoma parasitic worms, which can infect the urinary tract or the intestines and cause abdominal pain, diarrhea and blood in stool and urine.
The disease spreads through water contaminated with the parasites that host on freshwater snails, hence its nickname of ‘snail fever’.
According to the World Health Organisation (WHO) 40 million people were treated for schistosomiasis in 2013 while at least 261 million people required preventive treatment. Children are particularly vulnerable and it is found predominantly in Asia, Africa and South America.
However, DSM said the recommended drug praziquantel was often ineffective and repeat treatment risked the parasite becoming resistant.
As a result it said replacement or adjunctive strategies were needed.
The company referenced research in school children from El Kafr Sheikh in Egypt, which it said showed the omega-6 fatty acid ARA killed juvenile and adult S. masoni and S. haematobium worms.
Children already infected were given either a single dose of praziquantel then placebos for three weeks, ARA only or finally a combination of ARA and praziquantel.
The study used DSM's product Ig VegCap.
The results showed the combination group had the highest rate of eradication in cases of low, moderate and high infection at 74, 73 and 84%, respectively. For the Praziquantel-placebo group these rates were 57, 70 and 81%, respectively. For the omega-6 alone this was 50, 49 and 65%, respectively.
The patent proposed doses from five mg per kg body weight per day up to 600 mg per kg body weight per day over a period of between one and 30 days.
The omega-6 could come from a variety of sources including aquatic animals like fish and crustaceans, plant sources like corn and flaxseeds or microorganisms like microalgae and fungi.
In 2012 Cargill won EU novel food approval for algae fermented ARA-rich oil, with the main aim of using the ARA in infant and follow-on formula.
ARA is commonly found in such products alongside omega-3 form docosahexaenoic (DHA) and is found naturally in breast milk.
Source: WIPO Publication No. WO/2015/123480
Published: 20.08.2015 Filed: 13.02.2015
“Compositions and methods for the prevention and/or treatment of schistosomiasis"
Authors: DSM Assets – K. Hadley, R. El Ridi