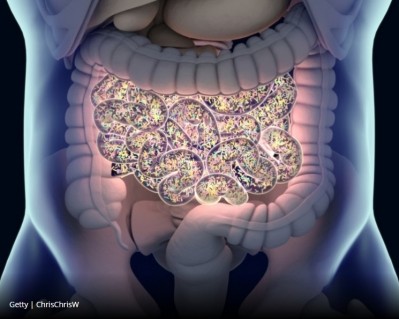
Baby faeces’ ‘good bacteria’ a solid basis for disease-tackling probiotic supplement

The findings identify these gut bacteria strains as crucial in assisting the body's ability to produce short-chain fatty acids (SCFAs) that are in short supply in those suffering from chronic conditions.
“Short-chain fatty acids are a key component of good gut health," said the study's lead investigator, Dr Hariom Yadav, assistant professor of molecular medicine at Wake Forest School of Medicine in North Carolina.
"People with diabetes, obesity, autoimmune disorders and cancers frequently have fewer short-chain fatty acids. Increasing them may be helpful in maintaining or even restoring a normal gut environment, and hopefully, improving health."
As the researchers state, infant faeces is preferred in research of this nature not only because the babies are in good health with an absence of age-related diseases but also because the team can rely on a plentiful supply of the waste material.
With much research, identifying probiotic strains’ ability to influence inflammatory bowel disease, obesity/type 2 diabetes, and autoimmunity, demand for probiotic supplements over the last decade has rocketed.
The development of new probiotic products for the consumer market has increased dramatically, with recent food launches such as Solgar Ohso Good Chocolate claiming to deliver probiotic bacteria three times more effectively than dairy products.
Other products include offerings by PepsiCo-owned Tropicana, which launched a range of probiotic juices in the US, containing the probiotic strain Bifidobacterium lactis.
While the positive modulation of gut microbiome has been suggested as one of the mechanisms underlying probiotic effects, how and why probiotics affect the gut microbiome and SCFA spectrum in healthy hosts still remains debatable.
Study details
Dr Yadav's team amassed faecal samples from 34 healthy infants, which then underwent isolation, characterization and safety validation of infant gut-origin Lactobacillus and Enterococcus strains with probiotic attributes. The team then selected the 10 best out of the 321 analysed.
Mice were given a single probiotic dose, as well as five consecutive doses of this 10-strain probiotic cocktail.
The team then injected the same probiotic mixture in the same doses into a human faeces medium.
Findings revealed that the single- and five-dose feeding of these selected probiotics modulated the gut microbiome and enhanced the production of SCFAs in mouse gut and human faeces.
"This work provides evidence that these human-origin probiotics could be exploited as biotherapeutic regimens for human diseases associated with gut microbiome imbalance and decreased SCFA production in the gut," Dr Yadav said.
"Our data should be useful for future studies aimed at investigating the influence of probiotics on human microbiome, metabolism and associated diseases."
Increase in SCFAs
While the changes in the microbial diversity were limited, the team were encouraged by the significant increase in SCFAs levels in the mouse gut.
Probiotic treatment however significantly enhanced microbial diversity and increased SCFA production in human faecal microbiobe.
“Feeding of probiotics (mostly single strain) induces minimal microbiome changes but can still enhance microbial diversity to produce beneficial metabolites i.e., SCFAs in the gut,” the study highlighted.
“Our results show that a probiotic cocktail containing multiple strains can cause a considerable shift in the gut microbiome signature, with increased SCFAs production.
Abnormally high Firmicutes and low Bacteroidetes abundances are associated with imbalanced microbiome composition (gut dysbiosis) and several dysbiosis-associated diseases including diabetes, obesity, cancer and irritable bowel disease.
“Although lactobacilli and enterococci both belong to Firmicutes, inoculation of these probiotics overall decreased Firmicutes and increased Bacteroidetes abundances in mice and human faecal microbiome.”
The team concluded that treatment with such human origin-probiotics might help enhance gut microbiome dysbiosis by enhancing microbial diversity, Bacteroidetes abundance, and SCFAs production, while suppressing Firmicutes population.
"In this study we tested in healthy mice and healthy human faecal suspension," said Dr Yadav. "Our next step will be to test them in different diseased models, as well as prepare formulations, that can be adopted for clinical studies."
In further discussing future directions, Dr Yadav said there were plans to patent the combinations of different strains for different applications adding that they were on the lookout for industry partners to work with and take the probiotics to market.
Source: Scientific Reports
Published online ahead of print: DOI: 10.1038/s41598-018-30114-4
“Human-origin probiotic cocktail increases short-chain fatty acid production via modulation of mice and human gut microbiome.”
Authors: Ravinder Nagpal, Shaohua Wang, Shokouh Ahmadi, Joshua Hayes, Jason Gagliano, Sargurunathan Subashchandrabose, Dalane W. Kitzman, Thomas Becton, Russel Read & Hariom Yadav

![Katherine Pollard (left) and Patrick Bradley (right) identified genes that may help microbes live successfully in the human gut. [Photo: Elisabeth Fall]](/var/wrbm_gb_food_pharma/storage/images/_aliases/wrbm_medium/publications/food-beverage-nutrition/nutraingredients.com/article/2018/08/14/survival-of-the-fittest-gut-colonisation-study-could-lead-to-gene-targeting-probiotics/8503315-1-eng-GB/Survival-of-the-fittest-Gut-colonisation-study-could-lead-to-gene-targeting-probiotics.jpg)




