Recall round-up: Moringa, CBD and multivitamin supplements

The Health Inspectorate of the Republic of Slovenia has taken steps to recall a Moringa dietary supplement owing to the high content of ethylene oxide.
Instructed by the Slovenian distributors, ‘Be Healthy doo Kranj (FutuNatura),’ the products consist of Moringa powder (500g, shelf life: 12.2.2023, Article number: 55) and Moringa capsules (240 capsules, shelf life: 28/29/30/31.10.2022 and 22.3.2023, Article number: 56).
Further details supplied by the Authorities identify the products’ supplier as Krauterhaus Sanct Bernhard, Bad Ditzenbach in Germany.
“Consumers who have already bought these supplements are advised not to consume them and to return them to the point of sale or. Discard,” they add.
Orion Pharma
Meanwhile over in Finland, pharmaceutical firm Orion Pharma has withdrawn its Multivita Forte food supplement from pharmacy shelves, again due to an excess of ethylene oxide levels.
In a press release issued by the firm, consumers have been asked to return opened and unused Multivita Forte products to the nearest pharmacy by the end of August 2021, where a refund will be made.
“The withdrawal is due to the ethylene oxide content of the ginger extract used in the Multivita Forte product exceeding the EU limit value,” the firm stated.
The withdrawal of these supplements comes after European countries issued a raft of ethylene oxide related recalls after the substance was detected in a food additive.
The compound was recently found in the additive locust bean gum, a thickening agent or stabiliser used in foods like confectionery, fermented milk products and cheese.
While consumption of certain foods containing this compound doesn’t present an acute risk to health, the risk is increased if contaminated foods are eaten over the long term with officials uncertain when contamination started.
THC & Turmeric
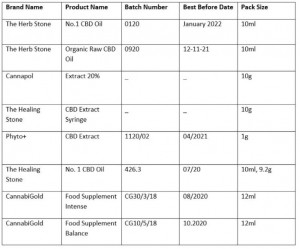
Finally, Ireland’s Food Safety Authority (FSAI) has distributed a couple of recall alerts, one for CBD-based dietary supplements that exceed safe levels of the psychoactive cannabis constituent, delta‐9‐tetrahydrocannabinol (THC).
Listing the implicated batches, the FSAI instructs distributors to withdraw the product from the market, whilst retailers are requested to remove the products from sale.
“Food businesses that have sold the implicated batches of the implicated CBD products to consumers must display a point-of-sale notice in store and on websites if sold online, to inform consumers that the implicated batches of these CBD products are being recalled and the reason why,” the Authority adds.
Ireland’s Food Authority also notifies consumers of a faulty batch of Organic Turmeric Powder due to elevated levels of Aflatoxin.
Analysis of the powder, made by True Natural Goodness, reveals elevated levels of the group of carcinogens and mutagens (mycotoxins) produced by certain moulds.
Mycotoxins can cause adverse health effects including cancer, kidney and liver damage, gastrointestinal disturbances, reproductive disorders, or suppression of the immune system.
Manufacturers, wholesalers, distributors, caterers & retailers are advised to remove the implicated batches from sale and to display a point-of-sale recall notice in stores where the affected batches were sold. Meanwhile, consumers are advised not to eat the implicated batches.