'Pioneering' study reveals collagen peptide changes during digestion
Collagen peptides have been shown to provide multiple benefits, especially in supporting skin, bone sports recovery and joint health. However, in order to fully understand the cellular mechanisms responsible for those effects, it’s important to map out the significant modifications that collagen peptides undergo when they pass the human digestion and are taken up by the body.
The new study, published in the journal Analytical and Bioanalytical Chemistry, investigates the transformation that different types of collagen peptides undergo during digestion and absorption. Confirming the findings of previous studies on bioavailability, it provides a useful snapshot of the changes the tested collagen peptides undergo during the course of digestion and absorption.
Janne Prawitt, Scientific Director Health and Nutrition at Rousselot, explains: "A key question amongst scientists and consumers is, ‘how does it work?’ There's a dogma around the digestibility of proteins, however there is still uncertainty over their bioavailability after digestion.
"This study takes a pioneering approach to capture the changes that Peptan undergoes while passing through our digestive tract and being absorbed into the blood stream. It provides invaluable new data that contribute to build up our science knowledge behind the health benefits of collagen peptides.
"This type of research is also essential as it help us create a complete and solid understanding of our collagen products in order to keep progressing and innovating as the leader of collagen-based solutions."
Methodology
The study was carried out in partnership with the French National Institute for Agricultural, Environment and Food Research (INRAe) and Triskelion, which offers high-end analytical research and compliance and risk management services.
Combining preclinical and clinical approaches, the study aimed to mimic the process of human digestion and absorption on four different types of collagen peptides. Provided by Rousselot, the initial products were of different animal sources and varying molecular weight.
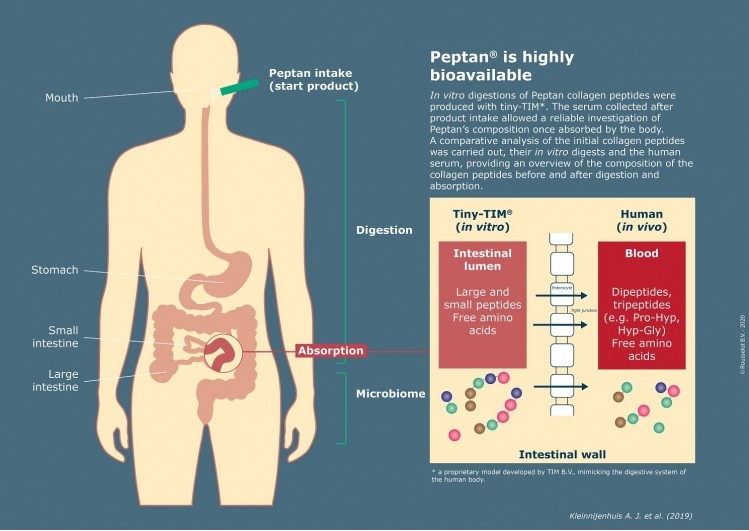
In vitro digestions of the four collagen hydrolysates were performed with the model system tiny-TIM (proprietary to TIM BV in the Netherlands), a state-of-the-art technology mimicking the chemical, kinetic and dynamic conditions of human digestion. It allows to identify the nutrient fractions that are available for absorption in the small intestine, providing valuable information on the bioavailability of collagen peptides.
INRAe performed a clinical test with 12 human volunteers who ingested 25g of collagen peptides. The serum collected after product intake allowed a reliable investigation of Peptan’s composition once absorbed by the body.
Triskelion carryied out a comparative analysis of the initial collagen peptides, their in vitro digests and the human serum, providing an overview of the composition of the collagen peptides before and after digestion and absorption.
Findings
Results showed that the tested collagen peptides underwent significant change during digestion and absorption. The average molecular weight decreased as the products were exposed to digestion enzymes and broken into smaller peptides. It was detected that the products of different animal sources become more similar when the average molecular weight drops along the digestion and the absorption process.
The human serum analysis revealed the presence of bioactive hydroxyproline-carrying dipeptides in the blood after Peptan ingestion. These dipeptides (Hydroxyproline-Glycine and Proline-Hydroxyproline) significantly contributed to the total increase of hydroxyproline, the characteristic amino acid of collagen, in the blood.
"At Rousselot, we understand the importance of reassuring consumers of the bioavailability of products and so we are increasingly making an effort to carry out these types of studies to assure people of the quality of our product."
Source: Analytical and Bioanalytical Chemistry
Kleinnijenhuis. A. J., et al
"Non-targeted and targeted analysis of collagen hydrolysates during the course of digestion and absorption"