George Paraskevakos, executive director at the IPA, pointed out regulatory challenges that were faced by the probiotics industry and offered potential solutions during his presentation at Probiota Asia last week.
The association has proposed to harmonise probiotics regulations at the CODEX level last year.
Argentina stepped up as lead nation for the work, with help from the IPA in drafting the proposal. IPA will be discussing the proposal with Argentina again next month.
“…The first thing that has to happen is that the community has to accept this new work from Argentina, take it on, and then from there, that’s where IPA gets involved to help to kind of shape the future in harmonisation which makes sense at the global level,” Paraskevakos said.
“We are really the ambassadors and the gatekeepers to help the industry thrive and grow.”
Explaining the need to improve regulatory system, he said that globally, “science and technology is marching on”, however, “regulations seem to be forever in flux.”
He thus suggested that guidelines keep up to pace with technology and science.
“Any type of harmonisation needs to be dynamic and open to modification with technology and research.”
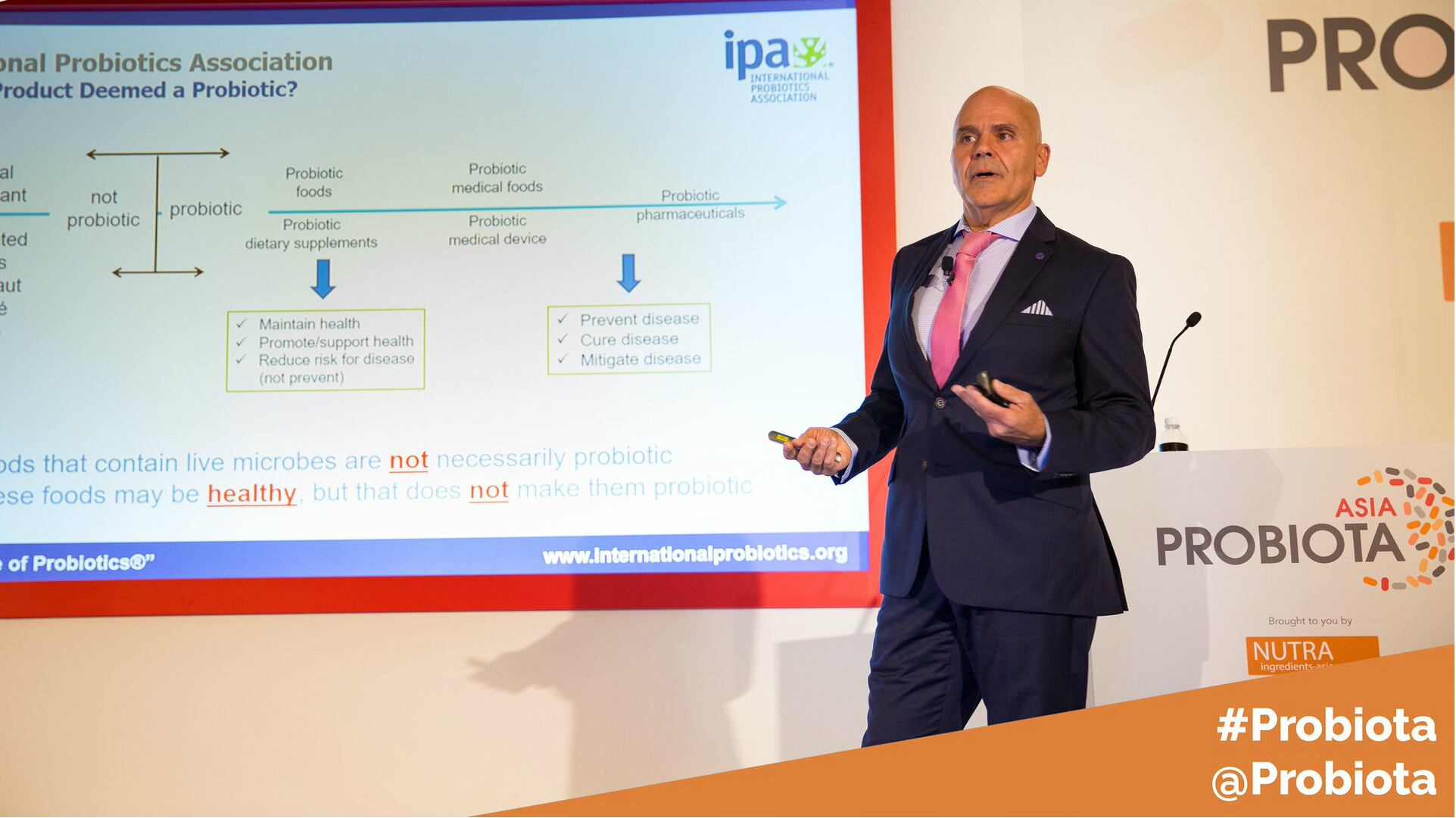
Currently, the accepted and respected definition for probiotics was established by an FAP/WHO expert panel in 2001/2002. However, it only provides general guidance for the recognition of health benefits of probiotics products.

NutraIngredients, in conjunction with GOED, will be holding APAC's first ever omega-3 event in Singapore in February 2019, featuring renowned speakers from CSIRO, A*STAR, Koure, GOED and more.
Country-specific regulations
At present, the probiotics industry faces the challenge of having to navigate through different regulations that are country specific.
“Definitions are country specific, which is the cause for confusion,” Paraskevakos said.
He cited several examples around the globe to illustrate the regulatory differences – ranging from labelling claims to approved ingredients.
The EU is a classic example. While products cannot be labelled as “probiotics” in the region, the term can be used in individual countries within the region, including Italy.
In the case of China, manufacturers will need to refer to the positive list and to go through the blue hat registration route to sell supplements within the country.
While in ASEAN, each country also has its own unique sets of regulations.
“A particular situation in Malaysia does not necessarily mean that these requirements will be the same as Cambodia, or Laos, Vietnam or Thailand. So (there is) further disparity, further gap, and further confusion.”
Most important ingredient
Probiotics was the fastest growing supplements globally in terms of market size last year.
It has consistently outpaced the growth of vitamins and other supplements since 2015, Paraskevakos pointed out.
The total global probiotic market is valued at around US$42.5bn, of which, $17.5bn comes from APAC.
A further breakdown showed that China contributed to almost half of the probiotic sales in APAC.
Yogurt was the most popular product format, responsible for 73% of the global retail last year, followed by fermented milk (15%), and supplements (12%), according to figures from Euromonitor.