EFSA sets ‘conservative’ safe intake level for DHA
EFSA has published its scientific opinion on the safe level of supplemental docosahexaenoic acid (DHA) following concerns that sustained use could increase the risk of spontaneous bleeding.

EFSA has published its scientific opinion on the safe level of supplemental docosahexaenoic acid (DHA) following concerns that sustained use could increase the risk of spontaneous bleeding.

The Alliance for Natural Health USA (ANH-USA) has filed a federal lawsuit against the U.S. Food and Drug Administration (FDA) over the agency’s recent rejection of a petition to allow over 110 ‘government-backed’ disease risk reduction health claims.

Scott Bass is launching a new firm—Scott Bass Life Sciences LLC—focused on two core offerings: high level strategic guidance for the global self-care sector and a low-cost dispute resolution model designed specifically for life sciences companies.

Day two at the IPA World Congress + Probiota Dublin 2026 will see experts discuss regulatory updates, postbiotic science and strain-level innovation.

Guest article
The Make America Healthy Again (MAHA) movement is built on a simple but powerful promise. Our government should expand access to preventive and wellness tools and reject one-size-fits-all bureaucratic solutions that treat citizens as incapable of making...


As of March 1, health food products containing coenzyme Q10 or melatonin as a single active ingredient will be permitted in oral liquid form in China.

U.S. Senate Democratic Whip Richard Durbin (D-IL) has introduced the Dietary Supplement Listing Act of 2026, which would require dietary supplement manufacturers to list their products with the Food and Drug Administration (FDA).

Delivery formats are becoming a growing compliance risk under DSHEA, warns industry attorney Jennifer Adams.

A lawmaker in Alaska has pre-filed a bill to prohibit the sale of weight loss and muscle building products to minors.



The CPG major faces the biggest product recall in its history - and investors want answers

During a press conference Wednesday, Health Secretary Robert F. Kennedy Jr. announced new dietary guidelines that flip the traditional food pyramid on its head.

Nestlé faces global scrutiny as infant formula recall widens over contamination fears

The U.S. Food and Drug Administration (FDA) has denied a petition from the Alliance for Natural Health USA (ANH-USA) seeking to allow over 115 ‘government-backed’ disease risk reduction health claims.


Do you have an initiative, program or project that produced significant improvements for the wider industry? If yes, NutraIngredients USA wants to hear from you for its Editors’ Award for Industry Initiative of the Year.

From product labeling to Good Manufacturing Practices requirements, new major regulations are expected to affect the nutraceutical industry across Asia-Pacific in 2026.

Amazon’s cGMP requirement for all health supplement products, Australia’s Therapeutic Goods Administration approval of NMN for use in dietary supplements, and the expansion of non-novel probiotic strains by the European Commission are some of the latest...

Tech giant Amazon is rolling out its requirements for all dietary supplement sellers on the platform to use a third-party testing, inspection, and certification (TIC) organization to demonstrate cGMP compliance, according to the American Herbal Products...

Banned Substances Control Group’s Oliver Catlin recently partnered with the Associated Press to expose something shocking: research peptides marketed as “laboratory chemicals” are flooding mainstream platforms like Amazon and Alibaba.

Guest article
For nine decades, the Natural Products Association has occupied a singular role within the dietary supplement industry: When its businesses are threatened, NPA fights.

The European Commission has updated its Novel Food Catalogue to add 34 probiotic strains considered ‘not novel’ when used in food or food supplements.

Research shows that some fish oil supplements are oxidized, but is there any scientific evidence to support the perception that oxidized fish oil is less efficacious or even detrimental?

As 2025 draws to a close, its clear this was a pivotal year for sports and active nutrition.

The Trump Administration issued an executive order today directing federal agencies to finalize the rescheduling of marijuana to Schedule III and to develop a new regulatory framework for hemp-derived cannabinoid products, including CBD.

South Korea’s Ministry of Food and Drug Safety (MFDS) has blocked online advertisements featuring AI-generated advertisements of doctors making misleading product claims, such as how nicotinamide mononucleotide (NMN) supplementation can promote cell...

Guest article
As the American Herbal Products Association (AHPA) readies for the new year, we pause to reflect on a period of profound change and robust growth, navigating an increasingly complex landscape of regulation, market trends and policy shifts. This year, we...

We enjoy writing some articles more than others, whether it’s because of the subject, the impact for industry, the insights from commentators or other reasons. But which articles did we enjoy writing the most and why?

Turmeric- or curcumin-containing natural health products (NHPs) in Canada must update their labels to warn about the risk of hepatotoxicity, following a review by Health Canada of the available information.


In a letter to the dietary supplements industry, the FDA’s deputy commissioner for human foods says the agency is considering a regulatory amendment regarding requirements around the DSHEA disclaimer on product labels.

Guest article
Transparency has suddenly become one of the most talked-about themes in today’s food and consumer safety debates. Policymakers are pressing for more visibility into the ingredients entering the food supply, and the momentum behind reforming the...

The Advertising Standards Authority (ASA) announced several rulings against supplement brands targeting Autistic consumers and those with attention deficit hyperactivity disorder (ADHD).

The IPA World Congress + Probiota 2026 is just two months away! The leading event for the probiotics, prebiotics and microbiome community is a must-attend event, and let us tell you why…

The U.S. Food and Drug Administration has sent letters to specific NMN ingredient suppliers confirming it has set aside previous determinations that the vitamin B3 form is excluded from the definition of a dietary supplement.

Guest article
What a year of transition, uncertainty and unpredictability 2025 has been—not just for the dietary supplement industry, but for all of us navigating rapid change. From shifting tariff rates to upheaval at FDA, from renewed pushes to restrict access to...

The National Institutes of Health has launched a five-year project to develop a unified model of healthy human physiology, signaling a shift toward whole-person health that aligns with longstanding approaches in the nutrition and supplements sector.

Tariffs meant to boost U.S. manufacturing might actually be pushing companies away from American investment. Chase Johnston, vice president of operations at Arjuna Natural, breaks down how rapidly shifting tariff rates are creating chaos for the...

The most popular articles on NutraIngredients in Europe this Fall highlighted how science, regulatory bodies, media scrutiny and increasingly educated consumers are shaping safety, searches, claims and trends in the supplement market.
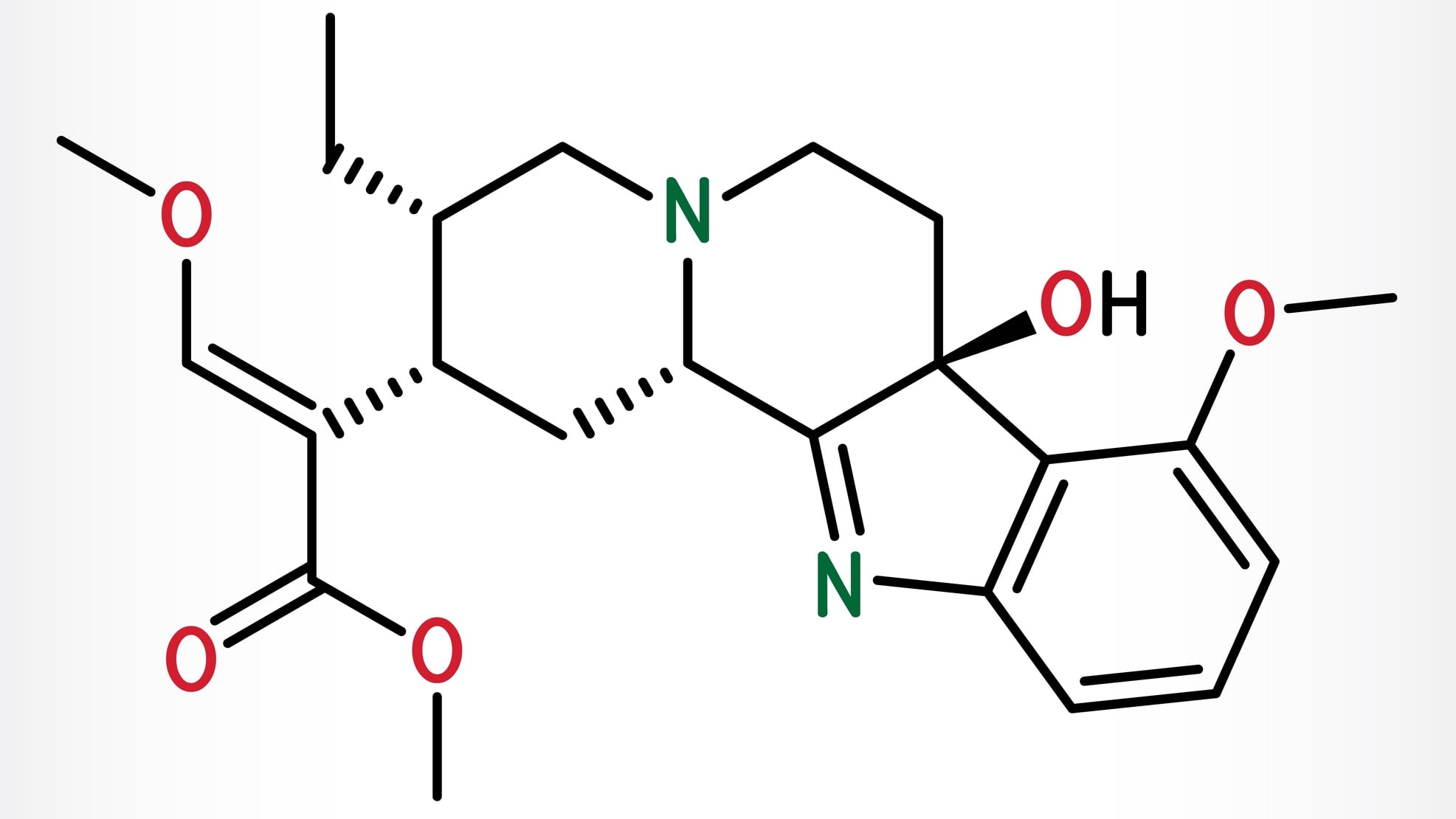
The U.S. Marshals Service has seized approximately 73,000 units of 7-hydroxymitragynine (7-OH) products from three firms in Missouri, the U.S. FDA announced.
Hear from experts from Wageningen University and myota in a free webinar on Dec. 9 that explores the challenges and opportunities of a growing prebiotic category.

Catch up with our weekly round-up of key news from across the Nutraverse.

The Protein Brewery is celebrating a positive scientific opinion from Europe’s food safety agency. So when will its new mycoprotein ingredient hit the market?

Australia’s implementation of ‘pharmacist-only’ scheme for vitamin B6, the US expanding tariff exemption list to include botanicals like turmeric, and the International Probiotics Association’s efforts in addressing expected changes to the USFDA GRAS...

The Council for Responsible Nutrition (CRN) has determined a Highest Observed Intake (HOI) level for vitamin K2-MK7 of 375 mcg/day following a review of over 40 clinical trials.


Vitamin B6 supplements with a recommended daily dose of over 50mg but 200mg or less will be sold as ‘pharmacist-only’ products in Australia from June 2027.