Trained in nutrition science and epidemiology at Wageningen University, Alwine Kardinaal now focuses on scientific substantiation of health benefits of foods and food ingredients in her role as expertise group leader, Nutrition & Health, at the food research firm NIZO.
How do we study the immune-boosting effect of ingredients and components in humans?
Alwine Kardinaal: The human immune system is very complex, involving the interaction of many cells and tissues in the body. In fact, the immune system has two distinct parts, working closely together: the innate immune system, which regulates the body’s short-term response to an external invader, and the adaptive immune system, which memorises the response and regulates the long-term reaction. It is important to know which part is relevant to your ingredient or component.
To understand and verify the immune-boosting effect of a food component, you need to collect strong evidence: not just of what the effect is, but how and why it occurs. This is best achieved with an integrative approach including pre-clinical studies, well-designed clinical trials, biomarker analysis and expert interpretation.
Clinical studies with faecal samples can provide insight on the impact of food components, fibres, probiotics and other ingredients on gut microbiota, and subsequent local and systemic immune responses.
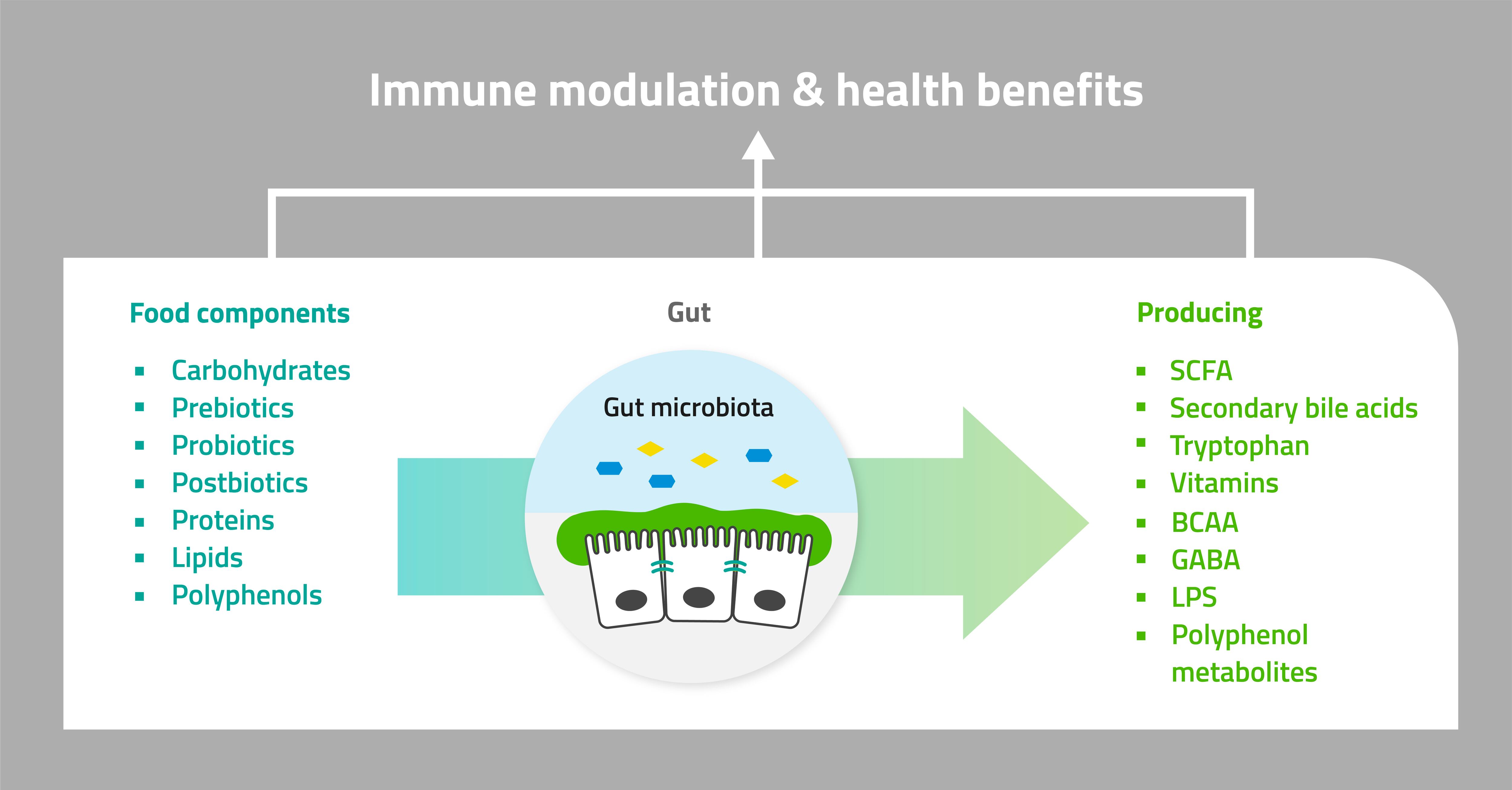
What are we specifically looking for in a clinical trial?
AK: A human clinical study on immune health benefits provides scientific evidence of whether the food component impacts resistance to infection. This could be either reducing the likelihood of infection itself, or reducing the severity or duration of the infection.
Three common approaches to human intervention studies include: field trials, infection challenges and vaccination trials. All of these involve randomised and placebo-controlled clinical trials.
How do you select which clinical trial approach to use?
AK: There are many aspects to take into account, including both the relevant food component and pathogen: some approaches are not appropriate for certain pathogens. Timeframe and cost can also be significant considerations, when the company wants to get their product to market quickly. But it is always important to consider the value of the trial, rather than the cost: what information will you get?
Pre-clinical and proof-of-concept phases can supply critical information for choosing a human intervention trial approach. In vitro and ex vivo studies can provide data on immune markers such as cytokine production, changes in cell numbers, phagocytosis activity, differential gene expression and much more. They also enable a comparison of the potential of different ingredients to impact specific mechanisms of action (i.e., how the ingredients work in the body); information on mechanisms of action may additionally be gained through preliminary, exploratory clinical studies.
Combining this insight with expert interpretation can provide direction for designing an approach that will increase the likelihood of getting compelling evidence from the clinical trials.
Combining a systematic approach with expert advice can help determine the most appropriate and cost-effective route to ingredient development, including which pre-clinical and clinical trials to use.
Field trials are often considered the ‘standard’. What do these involve, and what are the advantages and disadvantages?
AK: In field trials, subjects are given either the component or the placebo, then we wait for them to be spontaneously exposed to the pathogen in real-life situations. The study population may be selected from people at ‘high risk’ of pathogen exposure: adults who will be travelling to a region where food-borne infections are common (the dreaded ‘traveller’s disease’), children in day-care, or the elderly in a care-home. The subjects are followed for a period of time, and any differences in terms of incidence, severity and/or duration of infection between the placebo/food component groups are measured.
The advantage of this method is that you are studying people in real-life situations. However, you can’t know the rate of exposure to the target pathogen. You therefore need a larger sample size, for the statistical analysis. Furthermore, it can be difficult to determine how long to run the trial, to ensure you see a true effect from your component.
What is a challenge trial, and how does it compare to the field trial?
AK: In challenge trials, subjects are directly exposed to a relatively mild or attenuated pathogen. For example, at NIZO, we collaborate with Amsterdam UMC on a mild, low-contagion rhinovirus strain. We also have our own attenuated E. coli strain.
The challenge-based approach gives more control over the process. As all study participants are exposed to the pathogen at known times, we can use fewer test subjects, determine the length of the clinical trial, and sample at the most relevant moments. All of this reduces costs and timelines, compared to larger field studies. On the other hand, not every pathogen is appropriate for a challenge study, due to health and safety considerations.
Vaccination trials are a specific type of challenge trial: what are they used for?
AK: In a vaccination study, we are determining antibody response to the component. The subject is given a vaccine, with blood samples taken beforehand, and then at specific times afterwards to measure the increase in antibody titres. Outcomes can include vaccine-specific immunoglobulins, including IgA and IgG, the percentage of (non)responders, inflammatory markers such as cytokines and calprotectin, and more.
The subjects are not directly exposed to a pathogen, and so don’t exhibit symptoms. This means you cannot assess the impact of your component on the duration and severity of the infection. Furthermore, this type of study is only applicable for studying the adaptive immune system.
How do you select the target group?
AK: Ideally, we want a representative yet homogenous mix of genders and age groups, but this isn’t always possible. For example, the elderly can have more undiagnosed underlying diseases, for example. On a practical level, with women subjects, it can be difficult to differentiate gastro-intestinal and menstrual symptoms, and collecting uncontaminated faecal samples can be more difficult.
However, in certain cases, we can extrapolate the results from the trial population to a specific target group. The functioning of the immune systems of the elderly is generally the same, although less responsive, for instance. The mechanism of action can play a significant role in determining the confidence with which we can extrapolate.
This is something to consider in the design phase, based on factors including the mechanism of action, intended market, time and cost constraints, etc. No two human intervention trials are exactly alike: this is why you need an integrative, expert approach for choosing appropriate models, approaches, designs and outcomes.

