Introduction: adaptogens
An ever-increasing number of active ingredients qualified as adaptogens are currently emerging from the nutraceutical market. And for a good reason: the great fame of some of them in traditional medicine and their pleiotropic health benefits on immunity, stress, and fatigue, make them highly successful and effective active ingredients.
But what is so special about adaptogens?
From a functional perspective, adaptogens were historically described as "medicinal substances which induce an increased state of nonspecific resistance in the body". They are plants or fungi that typically increase the body's resilience to external stress (physiological, emotional, or even physical) and promote an overall return of physiological functions to a state of homeostasis.
From a biochemical point of view on the other hand, research shows that adaptogens are often characterized by a diverse molecular profile that combines bioactive secondary metabolites (terpenes, phenols, alkaloids, etc.) and specific polysaccharides, mainly found in the plant’s roots (and sometimes the fruits).
Could it be that the outstanding pleiotropic effects of adaptogens are in fact due to their particularly diverse molecular profile compared to other medicinal plants? Although research has not yet sufficiently highlighted this aspect, it seems that molecular diversity might indeed be a crucial factor of adaptogenic efficacy.
The future of adaptogens lies in clinical nutrition
Far from traditional plant concoctions, modern mainstream herbalism fits in a capsule. Deeply inspired by modern pharmacology, the nutraceutical industry has a tendency to consider botanicals as a source of a unique physiologically active molecule. Phyto-nutraceutical active ingredients are now often made of extracted and purified bioactive molecules with high therapeutic potential, intended to produce powerful / acute and targeted effects on the body; a pharma-inspired solution that has proven to be remarkably effective in the treatment of many pathologies.
This pharmacological conception, however, also has its limitations; it does not allow to fully harness and exploit the therapeutic potential of complex bioactive natural matrices with pleiotropic effects such as those of adaptogens. And this is because phytoactive enrichment or purification leads to the loss of a fraction of the full molecular profile.
In that regard, an interesting scientific approach to understand adaptogenic botanicals would be through clinical nutrition. Considering the plant as a functional food source of multiple phytonutrients and preserving their naturally complex bioactivity could lead to a better understanding of their systemic effects on the human body. A case in hand would be that of ginseng.
The case of ginseng
Panax ginseng C.A. meyer - Illustration by Botalys
Korean ginseng (Panax ginseng C.A. Meyer) is undoubtedly the most recognized and studied adaptogen in the world and deserves special mention in this regard. Indeed, the precious root has been used for millennia in traditional Asian medicine and has forged itself a fine reputation as a panacea thanks to its physical and mental stimulation potential and rejuvenating / revitalizing virtues.
Recent research has made it possible to highlight a family of particularly bioactive / high therapeutic potential molecules in ginseng: ginsenosides. These steroidal-structured saponins demonstrate numerous health benefits, from nootropic effects to hypoglycemic action, as well as immunomodulating and even anti-tumor properties. In particular, the noble / rare fraction, which includes the most bioactive representatives of ginsenosides, seems to confirm / explain most of the physiological effects attributed to ginseng in the Asian tradition.
However, it seems that ginsenosides alone do not explain the unique properties of ginseng. The global enthusiasm around this extraordinary plant has indeed led to an abundance of scientific data related to its characterization and to the potential contribution of other bioactive compounds to its effects on human physiology. To support this, we established a small review of some of the most prominent adaptogenic health effects of ginseng polysaccharides recently highlighted by scientific research.
- Ginseng and the neurological sphere
Ginsenosides, and in particular CK, Rg1, Rg2, Rg3, Rg5, Rh2 and Rk1, are strongly involved in the neurological benefits of ginseng. Indeed, research reports their significant neuroprotective [1] and nootropic potential which both lead to a positive impact on symptoms related to stress [2], depression [3-5] and mental and metabolic fatigue [6, 7]. Some ginsenosides also improve memory and learning capacities and could improve sleep through GABAergic pathway activation [8-10]. Ginsenosides Rg1, Rg3, Rg5, Rk1 and Rh2 also have significant potential for the prevention and early treatment of neurodegenerative diseases such as Alzheimer's [11-13].
Although little research has been done in this context so far, the anti-fatigue effects of ginseng root polysaccharides / pectins have also been demonstrated in an animal model of Chronic Fatigue Syndrome [14, 15], often correlated with depression.
- Ginseng and the immune system
Several ginsenosides (including in particular Rg1, Rg3, Rh1, Rh2, Rh4, Rk1) also exhibit immunomodulating / immunostimulating [16], anti-inflammatory [17, 18] and cytotoxic / anti-tumor [19] properties and may even present therapeutic potential in the treatment of autoimmune diseases such as endometriosis [20, 21].
Furthermore, ginseng polysaccharides, and in particular their acid fraction (including ginsans), have been investigated for their immunomodulating / immunostimulating effects [22]. In addition to potentiation of the immune response by stimulating the activity of macrophages and their cytotoxic mediators [23] and by increasing the number of B and T cells [24], ginseng polysaccharides also have significant anti-inflammatory activity and antioxidant [25] and anti-adhesive properties [26].
- Ginseng and metabolic regulation
Ginsenosides, including Rb1, Rg1, Rg3, Rg5, Re, Rh2 and CK, have great potential for metabolic regulation and prevention and treatment of diabetes and its complications [27]. Among the main effects shown are insulin-resistant activity [28], regulation of blood glucose and lipids levels as well as anti-adipogenic / anti-obesity effects [29] linked to PPARγ receptors downregulation.
Panaxans, a family of 21 peptidoglycans in ginseng, have demonstrated dose-dependent hypoglycemic activity. Several studies have shown that intraperitoneal administration of panaxans A-U [30-34] in healthy mice induces a dose-dependent significant reduction of blood glucose level, as well as hypoglycemic properties.
- Ginseng and metabolism
Despite their demonstrated prebiotic and immunomodulating potential, the number of scientific studies on ginseng polysaccharides remains relatively limited. Often co-extracted with ginsenosides, they are nevertheless of great pharmacological interest; the regulatory effect of polysaccharides on the microbiota could indeed promote the biotransformation of precursors into rare ginsenosides, induce an improvement in their pharmacokinetic parameters and their intestinal absorption rate. These effects would therefore lead to a better systemic exposure of ginsenosides in the presence of polysaccharides [35, 36], particularly in the metabolic context [37].
In addition to polysaccharides, ginseng contains many other bioactive molecules with high therapeutic potential that would deserve further study. This is particularly the case for:
- gintonin, a protein with high therapeutic potential for degenerative neurological and motor diseases [38].
- polyacetylenes such as panaxynol and panaxydol, with anti-platelet aggregation [39] and cytotoxic action [40] respectively.
- alkaloids like choline, which shows positive effects on blood pressure, memory or fat accumulation [41].
To conclude, ginseng perfectly embodies what modern pharmacology has to learn from traditional medicine. Although each of its bioactive molecules have demonstrated high efficacy on their own, it is highly probable that the “true” adaptogenic benefits of ginseng are brought about by their combined effects and interactions.
Full-spectrum - the essence of adaptogenic benefits
Everything seems to point at the importance of preserving a full-spectrum molecular profile to achieve the complete adaptogenic health effects of ginseng. Moreover, could this also be the case for other botanicals that fit in the biochemical or traditional definition of adaptogens?
There is indeed an increasing amount of evidence that suggests that adaptogen-derived polysaccharides significantly contribute to their pleiotropic benefits. Table 1 summarizes some of the demonstrated health benefits of polysaccharides extracted from six adaptogenic plants.
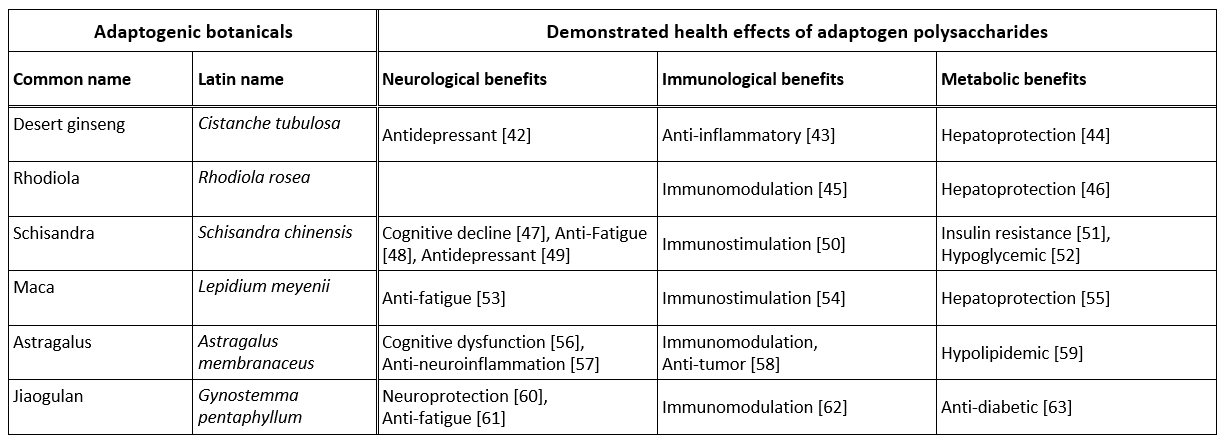
Table 1 - Health benefits of six adaptogenic plants polysaccharides
Polysaccharides are indeed the most glaring example of bioactive molecules that have remained in the shadow of secondary metabolites. They nevertheless demonstrate remarkable effects on the immune, metabolic and even neurological spheres. In addition, their prebiotic potential and their significant regulatory effects on the intestinal microbiota would have a crucial influence on the absorption and bioavailability of certain families of active molecules.
Beyond a direct physiological action through the involvement of secondary metabolites in various metabolic cascades, it would appear that the molecular diversity of the bioactive matrix, in particular specific polysaccharides, may also contribute to the systemic effects and broad-spectrum efficacy of adaptogens.
Adaptogens should no longer be regarded as a sum of active molecules, but rather as a complex matrix endowed with synergistic and multi-target properties, full of unsuspected potential for human health.
Time for a new standard
Adaptogens in general beautifully capture the complexity of the effects of herbal remedies on human physiology. Their pleiotropic effects suggest that the variety of bioactive natural molecules contained in the plant matrix are not only involved in a wide variety of metabolic cascades, but also that they may have combined / synergistic effects.
Even if the current state of research does not yet allow us to fully understand these synergies, research points at an increased bioavailability and effectiveness of full-spectrum (multimolecular) preparations of adaptogens compared to their purified extracts (monomolecular). The obvious parallelism between the spheres of action of secondary metabolites and plant polysaccharides strongly suggests an overlapping of their benefits.
The full potential of “true adaptogens” could therefore only be achieved with full-spectrum botanicals that preserve the complete molecular profile of the plant. Despite their demonstrated pharmacological efficacy, using extracts potentially means forgoing the complementary and / or combined effects of the various components of the plant.
But the varying quality of botanical extracts on the market makes it difficult to obtain solutions that are natural, effective and safe. Indeed, two major challenges linked to botanical extracts of agricultural origin remain: the significant risks of contamination from pesticides and heavy metals, as well as the considerable difficulty of standardizing (from one batch to another and over time) of raw materials of varying composition.
The transposition of the principles of phytotherapy to modern pharmacology, and in particular in a clinical context, thus requires the production of botanical species of pristine quality; a standard that seems unattainable for natural nutraceutical active ingredients from agriculture.
- Botalys: clinical grade full-spectrum adaptogens - phytotherapy 2.0
At Botalys, we believe the future of adaptogens lies in clinical nutrition. We have dedicated 10 years to exploring botanical biodiversity and building in-depth expertise on the therapeutic potential of their phytonutrients. We believe that harnessing the full potential of plants goes hand in hand with the utmost respect of their (original) nature, their environment and - most importantly - the consumers' health.
Botalys aims to push the boundaries of the world of medicinal plants and pave the way for a new standard in the nutraceutical sector to ensure the quality, efficacy, safety and sustainability of medicinal plants. By combining the full-spectrum approach with innovative indoor vertical farming technology, Botalys produces clinical-grade adaptogens that are naturally rich in a wide range of active substances while ensuring a very high degree of standardization and purity.
References
- [1] Cheng, Z., Zhang, M., Ling, C., Zhu, Y., Ren, H., Hong, C., ... & Wang, J. (2019). Neuroprotective effects of ginsenosides against cerebral ischemia. Molecules, 24(6), 1102.
- [2] Cha, H. Y., Park, J. H., Hong, J. T., Yoo, H. S., Song, S., Hwang, B. Y., ... & Oh, K. W. (2005). Anxiolytic-like effects of ginsenosides on the elevated plus-maze model in mice. Biological and Pharmaceutical Bulletin, 28(9), 1621-1625.
- [3] Zhang, H., Li, Z., Zhou, Z., Yang, H., Zhong, Z., & Lou, C. (2016). Antidepressant-like effects of ginsenosides: a comparison of ginsenoside Rb3 and its four deglycosylated derivatives, Rg3, Rh2, compound K, and 20 (S)-protopanaxadiol in mice models of despair. Pharmacology Biochemistry and Behavior, 140, 17-26.
- [4] Li, Z., Zhao, L., Chen, J., Liu, C., Li, S., Hua, M., ... & Sun, Y. (2020). Ginsenoside Rk1 alleviates LPS-induced depression-like behavior in mice by promoting BDNF and suppressing the neuroinflammatory response. Biochemical and Biophysical Research Communications, 530(4), 658-664.
- [5] Xu, D., Wang, C., Zhao, W., Gao, S., & Cui, Z. (2017). Antidepressant-like effects of ginsenoside Rg5 in mice: Involving of hippocampus BDNF signaling pathway. Neuroscience letters, 645, 97-105.
- [6] Yang, Q. Y., Lai, X. D., Ouyang, J., & Yang, J. D. (2018). Effects of Ginsenoside Rg3 on fatigue resistance and SIRT1 in aged rats. Toxicology, 409, 144-151.
- [7] Xu, Y., Zhang, P., Wang, C., Shan, Y., Wang, D., Qian, F., ... & Zhu, C. (2013). Effect of ginsenoside Rg3 on tyrosine hydroxylase and related mechanisms in the forced swimming-induced fatigue rats. Journal of ethnopharmacology, 150(1), 138-147.
- [8] An, K. S., Choi, Y. O., Lee, S. M., Ryu, H. Y., Kang, S. J., Yeon, Y., ... & Song, K. S. (2019). Ginsenosides Rg5 and Rk1 enriched cultured wild ginseng root extract bioconversion of Pediococcus pentosaceus HLJG0702: Effect on scopolamine-induced memory dysfunction in mice. Nutrients, 11(5), 1120.
- [9] Kim, J., Shim, J., Lee, S., Cho, W. H., Hong, E., Lee, J. H., ... & Lee, K. W. (2016). Rg3-enriched ginseng extract ameliorates scopolamine-induced learning deficits in mice. BMC complementary and alternative medicine, 16(1), 1-9.
- [10] Hou, J., Xue, J., Lee, M., Liu, L., Zhang, D., Sun, M., ... & Sung, C. (2013). Ginsenoside Rh2 improves learning and memory in mice. Journal of medicinal food, 16(8), 772-776.
- [11] Li, N., Liu, Y., Li, W., Zhou, L., Li, Q., Wang, X., & He, P. (2016). A UPLC/MS-based metabolomics investigation of the protective effect of ginsenosides Rg1 and Rg2 in mice with Alzheimer's disease. Journal of Ginseng Research, 40(1), 9-17.
- [12] Zhang, Y., Yang, X., Wang, S., & Song, S. (2019). Ginsenoside Rg3 prevents cognitive impairment by improving mitochondrial dysfunction in the rat model of alzheimer’s disease. Journal of agricultural and food chemistry, 67(36), 10048-10058.
- [13] Chu, S., Gu, J., Feng, L., Liu, J., Zhang, M., Jia, X., ... & Yao, D. (2014). Ginsenoside Rg5 improves cognitive dysfunction and beta-amyloid deposition in STZ-induced memory impaired rats via attenuating neuroinflammatory responses. International Immunopharmacology, 19(2), 317-326.
- [14] Wang, J., Sun, C., Zheng, Y., Pan, H., Zhou, Y., & Fan, Y. (2014). The effective mechanism of the polysaccharides from Panax ginseng on chronic fatigue syndrome. Archives of pharmacal research, 37(4), 530-538.
- [15] Wang, J., Li, S., Fan, Y., Chen, Y., Liu, D., Cheng, H., ... & Zhou, Y. (2010). Anti-fatigue activity of the water-soluble polysaccharides isolated from Panax ginseng CA Meyer. Journal of ethnopharmacology, 130(2), 421-423.
- [16] Christensen, L. P. (2008). Ginsenosides: chemistry, biosynthesis, analysis, and potential health effects. Advances in food and nutrition research, 55, 1-99.
- [17] Kim, J. H., Yi, Y. S., Kim, M. Y., & Cho, J. Y. (2017). Role of ginsenosides, the main active components of Panax ginseng, in inflammatory responses and diseases. Journal of ginseng research, 41(4), 435-443.
- [18] Im, D. S. (2020). Pro-Resolving Effect of ginsenosides as an anti-inflammatory mechanism of Panax ginseng. Biomolecules, 10(3), 444.
- [19] Jin, Y., Kim, Y. J., Jeon, J. N., Wang, C., Min, J. W., Noh, H. Y., & Yang, D. C. (2015). Effect of white, red and black ginseng on physicochemical properties and ginsenosides. Plant Foods for Human Nutrition, 70(2), 141-145.
- [20] Zhang, B., Zhou, W. J., Gu, C. J., Wu, K., Yang, H. L., Mei, J., ... & Li, M. Q. (2018). The ginsenoside PPD exerts anti-endometriosis effects by suppressing estrogen receptor-mediated inhibition of endometrial stromal cell autophagy and NK cell cytotoxicity. Cell death & disease, 9(5), 1-13.
- [21] Kim, M. K., Lee, S. K., Park, J. H., Lee, J. H., Yun, B. H., Park, J. H., ... & Choi, Y. S. (2017). Ginsenoside Rg3 decreases fibrotic and invasive nature of endometriosis by modulating miRNA-27b: in vitro and in vivo studies. Scientific reports, 7(1), 1-14.
- [22] Wee, J. J., Park, K. M., & Chung, A. S. (2011). Biological activities of ginseng and its application to human health. Herbal Medicine: Biomolecular and Clinical Aspects. 2nd edition.
- [23] Lim, T. S., Na, K., Choi, E. M., Chung, J. Y., & Hwang, J. K. (2004). Immunomodulating activities of polysaccharides isolated from Panax ginseng. Journal of medicinal food, 7(1), 1-6.
- [24] Youn, S. H., Lee, S. M., Han, C. K., In, G., Park, C. K., & Hyun, S. H. (2020). Immune activity of polysaccharide fractions isolated from Korean Red Ginseng. Molecules, 25(16), 3569.
- [25] Kim, H. M., Song, Y., Hyun, G. H., Long, N. P., Park, J. H., Hsieh, Y. S., & Kwon, S. W. (2020). Characterization and antioxidant activity determination of neutral and acidic polysaccharides from Panax ginseng CA Meyer. Molecules, 25(4), 791.
- [26] Lee, J. H., Shim, J. S., Lee, J. S., Kim, M. K., Chung, M. S., & Kim, K. H. (2006). Pectin-like acidic polysaccharide from Panax ginseng with selective antiadhesive activity against pathogenic bacteria. Carbohydrate Research, 341(9), 1154-1163.
- [27] Bai, L., Gao, J., Wei, F., Zhao, J., Wang, D., & Wei, J. (2018). Therapeutic potential of ginsenosides as an adjuvant treatment for diabetes. Frontiers in pharmacology, 9, 423.
- [28] Ponnuraj, S. P., Siraj, F., Kang, S., Noh, H. Y., Min, J. W., Kim, Y. J., & Yang, D. C. (2014). Amelioration of insulin resistance by Rk1+ Rg5 complex under endoplasmic reticulum stress conditions. Pharmacognosy research, 6(4), 292.
- [29] Simu, S. Y., Ahn, S., Castro-Aceituno, V., & Yang, D. C. (2017). Ginsenoside Rg5: Rk1 exerts an anti-obesity effect on 3T3-L1 cell line by the downregulation of PPARγ and CEBPα. Iranian journal of biotechnology, 15(4), 252.
- [30] Konno, C., Sugiyama, K., Kano, M., Takahashi, M., & Hikino, H. (1984). Isolation and hypoglycaemic activity of Panaxans A, B, C, D and E, Glycans of Panax ginseng Roots1. Planta medica, 50(05), 434-436.
- [31] Hikino, H., Oshima, Y., Suzuki, Y., & Konno, C. (1985). Isolation and hypoglycemic activity of panaxans F, G and H, glycans of Panax ginseng roots. 生薬学雑誌, 39(4), p331-333.
- [32] Oshima, Y., Konno, C., & Hikino, H. (1985). Isolation and hypoglycemic activity of panaxans I, J, K and L, glycans of Panax ginseng roots. Journal of ethnopharmacology, 14(2-3), 255-259.
- [33] Konno, C., & Hikino, H. (1987). Isolation and hypoglycemic activity of panaxans M, N, O and P, glycans of Panax ginseng roots. International Journal of Crude Drug Research, 25(1), 53-56.
- [34] Chohachi, K., Miki, M., Yoshiteru, O., & Hiroshi, H. (1985). Isolation and hypoglycemic activity of panaxans Q, R, S, T and U, glycans of Panax ginseng roots. Journal of ethnopharmacology, 14(1), 69-74.
- [35] Shen, H., Gao, X. J., Li, T., Jing, W. H., Han, B. L., Jia, Y. M., ... & Yan, R. (2018). Ginseng polysaccharides enhanced ginsenoside Rb1 and microbial metabolites exposure through enhancing intestinal absorption and affecting gut microbial metabolism. Journal of ethnopharmacology, 216, 47-56.
- [36] Liu, J., Li, T., Wang, J., Zhao, C., Geng, C., Meng, Q., ... & Yin, J. (2020). Different absorption and metabolism of ginsenosides after the administration of total ginsenosides and decoction of Panax ginseng. Rapid Communications in Mass Spectrometry, 34(13), e8788.
- [37] Li, J., Li, R., Li, N., Zheng, F., Dai, Y., Ge, Y., ... & Yu, S. (2018). Mechanism of antidiabetic and synergistic effects of ginseng polysaccharide and ginsenoside Rb1 on diabetic rat model. Journal of pharmaceutical and biomedical analysis, 158, 451-460.
- [38] Choi, S. H., Lee, R., Nam, S. M., Kim, D. G., Cho, I. H., Kim, H. C., ... & Nah, S. Y. (2021). Ginseng gintonin, aging societies, and geriatric brain diseases. Integrative Medicine Research, 10(1), 100450.
- [39] Teng, C. M., Kuo, S. C., Ko, F. N., Lee, J. C., Lee, L. G., Chen, S. C., & Huang, T. F. (1989). Antiplatelet actions of panaxynol and ginsenosides isolated from ginseng. Biochimica et Biophysica Acta (BBA)-General Subjects, 990(3), 315-320.
- [40] MATSUNAGA, H., Katano, M., Yamamoto, H., Fujito, H., Mori, M., & Takata, K. (1990). Cytotoxic activity of polyacetylene compounds in Panax ginseng CA Meyer. Chemical and Pharmaceutical Bulletin, 38(12), 3480-3482.
- [41] Hyun, S. H., Kim, S. W., Seo, H. W., Youn, S. H., Kyung, J. S., Lee, Y. Y., ... & Han, C. K. (2020). Physiological and pharmacological features of the non-saponin components in Korean Red Ginseng. Journal of Ginseng Research, 44(4), 527-537.
- [42] Bao, X., Bai, D., Liu, X., Wang, Y., Zeng, L., Wei, C., & Jin, W. (2021). Effects of the Cistanche tubulosa Aqueous Extract on the Gut Microbiota of Mice with Intestinal Disorders. Evidence-Based Complementary and Alternative Medicine, 2021.
- [43] Guo, J., Tang, S., Ge, L., Xu, J., & Zeng, X. (2021). The Anti-inflammatory Effects of Lignan Glycosides from Cistanche tubulosa stems on LPS/IFN-γ-induced RAW264. 7 Macrophage Cells via PI3K/AKT Pathway. Current Pharmaceutical Biotechnology.
- [44] Morikawa, T., Pan, Y., Ninomiya, K., Imura, K., Matsuda, H., Yoshikawa, M., ... & Muraoka, O. (2010). Acylated phenylethanoid oligoglycosides with hepatoprotective activity from the desert plant Cistanche tubulosa. Bioorganic & medicinal chemistry, 18(5), 1882-1890.
- [45] Cai, Z., Li, W., Wang, H., Yan, W., Zhou, Y., Wang, G., ... & Wang, F. (2012). Antitumor effects of a purified polysaccharide from Rhodiola rosea and its action mechanism. Carbohydrate polymers, 90(1), 296-300.
- [46] Xu, Y., Jiang, H., Sun, C., Adu-Frimpong, M., Deng, W., Yu, J., & Xu, X. (2018). Antioxidant and hepatoprotective effects of purified Rhodiola rosea polysaccharides. International journal of biological macromolecules, 117, 167-178.
- [47] Xu, M., Yan, T., Fan, K., Wang, M., Qi, Y., Xiao, F., ... & Jia, Y. (2019). Polysaccharide of Schisandra chinensis Fructus ameliorates cognitive decline in a mouse model of Alzheimer's disease. Journal of ethnopharmacology, 237, 354-365.
- [48] Chi, A., Zhang, Y., Kang, Y., & Shen, Z. (2016). Metabolic mechanism of a polysaccharide from Schisandra chinensis to relieve chronic fatigue syndrome. International journal of biological macromolecules, 93, 322-332.
- [49] Yan, T., Wang, N., Liu, B., Wu, B., Xiao, F., He, B., & Jia, Y. (2021). Schisandra chinensis ameliorates depressive‐like behaviors by regulating microbiota‐gut‐brain axis via its anti‐inflammation activity. Phytotherapy Research, 35(1), 289-296.
- [50] Zhao, T., Mao, G., Mao, R., Zou, Y., Zheng, D., Feng, W., ... & Wu, X. (2013). Antitumor and immunomodulatory activity of a water-soluble low molecular weight polysaccharide from Schisandra chinensis (Turcz.) Baill. Food and chemical toxicology, 55, 609-616.
- [51] Qiao, Z., Du, X., Zhuang, W., Yang, S., Li, H., Sun, J., ... & Wang, C. (2020). Schisandra chinensis acidic polysaccharide improves the insulin resistance in type 2 diabetic rats by inhibiting inflammation. Journal of medicinal food, 23(4), 358-366.
- [52] Du, X. X., Tao, X., Liang, S., Che, J. Y., Yang, S., Li, H., ... & Wang, C. M. (2019). Hypoglycemic effect of acidic polysaccharide from schisandra chinensis on T2D rats induced by high-fat diet combined with STZ. Biological and Pharmaceutical Bulletin, b18-00915.
- [53] Li, J., Sun, Q., Meng, Q., Wang, L., Xiong, W., & Zhang, L. (2017). Anti-fatigue activity of polysaccharide fractions from Lepidium meyenii Walp.(maca). International journal of biological macromolecules, 95, 1305-1311.
- [54] Wang, W., Zou, Y., Li, Q., Mao, R., Shao, X., Jin, D., ... & Wu, X. (2016). Immunomodulatory effects of a polysaccharide purified from Lepidium meyenii Walp. on macrophages. Process Biochemistry, 51(4), 542-553.
- [55] Zhang, L., Zhao, Q., Wang, L., Zhao, M., & Zhao, B. (2017). Protective effect of polysaccharide from maca (Lepidium meyenii) on Hep-G2 cells and alcoholic liver oxidative injury in mice. International journal of biological macromolecules, 99, 63-70.
- [56] Liu, Y., Liu, W., Li, J., Tang, S., Wang, M., Huang, W., ... & Gao, X. (2019). A polysaccharide extracted from Astragalus membranaceus residue improves cognitive dysfunction by altering gut microbiota in diabetic mice. Carbohydrate polymers, 205, 500-512.
- [57] Huang, Y. C., Tsay, H. J., Lu, M. K., Lin, C. H., Yeh, C. W., Liu, H. K., & Shiao, Y. J. (2017). Astragalus membranaceus-polysaccharides ameliorates obesity, hepatic steatosis, neuroinflammation and cognition impairment without affecting amyloid deposition in metabolically stressed APPswe/PS1dE9 mice. International journal of molecular sciences, 18(12), 2746.
- [58] Yu, J., Dong, X. D., Jiao, J. S., Ji, H. Y., & Liu, A. J. (2021). Antitumor and immunoregulatory activities of a novel polysaccharide from Astragalus membranaceus on S180 tumor-bearing mice. International Journal of Biological Macromolecules.
- [59] Pan, S., Gao, R., & Wu, S. (2017). Preparation, characterization and hypolipidaemic activity of Astragalus membranaceus polysaccharide. Journal of Functional Foods, 39, 264-267.
- [60] Jia, D., Rao, C., Xue, S., & Lei, J. (2015). Purification, characterization and neuroprotective effects of a polysaccharide from Gynostemma pentaphyllum. Carbohydrate polymers, 122, 93-100.
- [61] Qi, B., & Huang, H. (2014). Anti-fatigue effects of polysaccharides from Gynostemma pentaphyllum makino by forced swimming test. In Advanced Materials Research (Vol. 881, pp. 426-429). Trans Tech Publications Ltd.
- [62] Ren, D., Zhao, Y., Zheng, Q., Alim, A., & Yang, X. (2019). Immunomodulatory effects of an acidic polysaccharide fraction from herbal Gynostemma pentaphyllum tea in RAW264. 7 cells. Food & function, 10(4), 2186-2197.
- [63] Wang, Z., Zhao, X., Liu, X., Lu, W., Jia, S., Hong, T., ... & Zhan, X. (2019). Anti-diabetic activity evaluation of a polysaccharide extracted from Gynostemma pentaphyllum. International journal of biological macromolecules, 126, 209-214.


