There is a pressing need for alternatives to antibiotics in the prevention and management of urinary tract infections (UTIs). The rising global disease burden for UTI and knowledge that antibiotic use drives the emergence of drug-resistant bacteria has created a major opportunity for new ways of managing the common infection.1,2 Recognizing the unmet need, International Health Science (IHS) has developed an antibiotic-free, clinically validated product to support urinary tract health.
The product is aimed at a large market. In the US, the prevalence of UTI is 11% across the population and rises with age.3 Prevalence in women, who are more likely to have a UTI than men, aged over 65 years is 20%. Around 60% of women will suffer at least one UTI in their life. Almost one-in-10 postmenopausal women say they have experienced a UTI in the past year.
Similar trends are seen in Europe. Across the European Union, more than 4 million people contract healthcare-associated UTIs (HAUTIs) each year. At 19.6%, the prevalence of HAUTIs is higher in Europe than in the US, where the rate is 12.9%. The rate in developing countries is even higher.
UTIs are rarely life threatening but they place considerable burdens on patients and healthcare systems. The infections are associated with significant impacts on quality of life, with patients being forced to take sick leave, having days of limited activity and suffering mental stress.4 In the US, annual health costs for UTI exceed $1 billion (€907 million).5 Another study found the mean cost per case of complicated UTIs in eight European countries is €5,700.6
Antibiotics are routinely used to treat UTIs but they have downsides. After experiencing a UTI, 27% of women suffer a recurrence within six months, indicating the inability of antibiotics to consistently clear the infection permanently the first time.3 The recurrence of UTIs necessitates more antibiotic use, which creates further problems.
Repeated use of antibiotics is associated with the emergence of multidrug resistant pathogens and the dysbiosis of the microbiota.7 Dysbiosis is linked to recurrent UTIs, suggesting antibiotics may both treat the infection and raise the risk of recurrence.2,8 Rising rates of antimicrobial resistance are a serious threat to global public health.2,8
Identifying molecules to tackle UTI
The shortcomings of antibiotics mean that, while they will remain the main drugs to reduce infections, there is a recognised need for other interventions that can bypass drug resistance, enhance the innate immune response, preserve the microbiome and prevent the formation of biofilms. The problem is there has historically been a lack of clinically validated alternatives to antibiotics.
When researchers reviewed the UTI landscape in 2018, they found insufficient evidence to recommend a clutch of nonantibiotic options but saw sufficient promise to call for more trials.2,8 Two years later, IHS, then part of Giellepi, supported a preclinical evaluation of a panel of compounds, including a sugar monomer, D-mannose, that was recommended for further study in the 2018 review.9
The IHS-backed preclinical study evaluated two different combinations of molecules intended for use against acute and recurrent infections. With both sets of molecules having strengths and weaknesses, the researchers selected the best elements of each composition for evaluation in in vitro preclinical tests.
The result is a formulation known by the acronym DAPAD. The formulation consists of: D-mannose to enhance the protective biofilm; a standardized form of the medicinal herbs Astragalus membranaceus radix (AxtragylTM) for its anti-inflammatory and antioxidant properties; a proprietary prebiotic mix to boost the microbiota of the gut as well as the urogenital tract; citric acid for its antimicrobial effects; and dandelion for its anti-inflammatory and diuretic qualities.
Preclinical testing of DAPAD found the formulation inhibits growth of all UTI bacteria and elucidated the effects of the individual molecules, confirming the rationale for the combination.10 As well as inhibiting uropathogens, the combination reduced biofilm formation and had no manifested toxicity in bladder cell morphology. The preclinical results supported further development of DAPAD.
Validating DAPAD in humans
A randomised, double-blind, placebo-controlled clinical trial has now validated encouraging early data on DAPAD in humans. The study randomised 70 women with uncomplicated acute UTI to take two packets a day of DAPAD or placebo, dissolved in a glass of water, for five consecutive days.
One month after the end of treatment with DAPAD, 89% of women in the DAPAD cohort had no symptoms, compared to 20% of their peers in the control group (figure 1). The result caused the clinical trial to meet its primary endpoint. Secondary endpoint data provided more evidence of the effect of DAPAD on UTI.
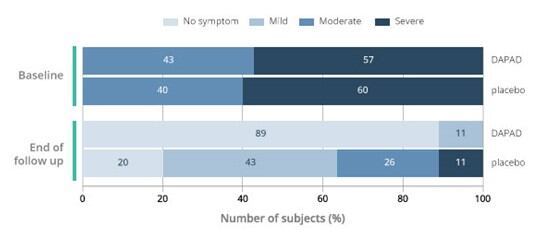
Figure 1. Severity of UTI symptoms in subjects treated with DAPAD or placebo at baseline and one month after the end of treatment (end of follow up). p<0.0001 DAPAD vs Placebo at end of follow up.
After five days of treatment, urine cultures were negative in 86% of subjects in the DAPAD cohort, compared to 14% of the placebo group, a statistically significant difference that indicates bacteriological resolution. IHS is now preparing the full data for publication while working to initiate a randomised clinical trial in women with recurrent UTIs.
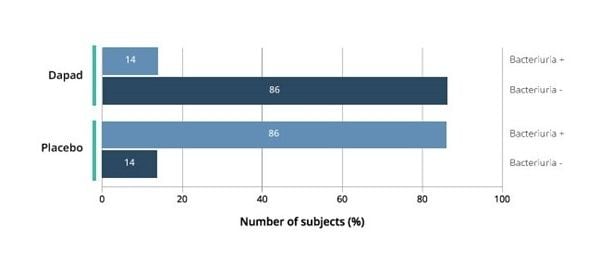
Figure 2. Bacteriuria resolution. 86% of subjects showed negative urine culture after 5-days treatment with DAPAD (p<0.001 vs placebo).
The clinical data position DAPAD as an answer to the unmet need in UTI. IHS, formerly Giellepi’s Finished Product business unit, is making the DAPAD sachets available for licensing as part of its focus on creating ready-to-market solutions.
IHS has developed DAPAD to address the needs of a new, fast-growing group of consumers who are taking a holistic view to their health and the impact of their actions on the planet. As well as having traditionally attractive attributes such as its clinical validation, fast onset of action and ease of use, DAPAD is free from gluten, lactose, artificial flavours and sweeteners, and animal products.
Those attributes, which make DAPAD vegetarian and vegan friendly, are particularly important given that women suffer more UTIs than men. As women are also more likely to avoid or eliminate animal products from their diets and to suffer from gluten intolerance, UTI products need to meet those needs to unlock the full market.11–13 Companies that licence DAPAD therefore stand to support the millions of women who need a non-antibiotic, clinically validated way to manage their urinary tract health.
Related
1. Zhu, C. et al. Epidemiological trends of urinary tract infections, urolithiasis and benign prostatic hyperplasia in 203 countries and territories from 1990 to 2019. Mil Med Res 8, 64 (2021).
2. Sihra, N., Goodman, A., Zakri, R., Sahai, A. & Malde, S. Nonantibiotic prevention and management of recurrent urinary tract infection. Nat. Rev. Urol. 15, 750–776 (2018).
3. Medina, M. & Castillo-Pino, E. An introduction to the epidemiology and burden of urinary tract infections. Ther. Adv. Urol. 11, 1756287219832172 (2019).
4. Wagenlehner, F., Wullt, B., Ballarini, S., Zingg, D. & Naber, K. G. Social and economic burden of recurrent urinary tract infections and quality of life: a patient web-based study (GESPRIT). Expert Rev. Pharmacoecon. Outcomes Res. 18, 107–117 (2018).
5. What is the economic burden of urinary tract infection (UTI)? https://www.medscape.com/answers/452604-54604/what-is-the-economic-burden-of-urinary-tract-infection-uti (2021).
6. Vallejo-Torres, L. et al. Cost of hospitalised patients due to complicated urinary tract infections: a retrospective observational study in countries with high prevalence of multidrug-resistant Gram-negative bacteria: the COMBACTE-MAGNET, RESCUING study. BMJ Open 8, e020251 (2018).
7. Jones-Freeman, B. et al. The microbiome and host mucosal interactions in urinary tract diseases. Mucosal Immunol. 14, 779–792 (2021).
8. Josephs-Spaulding, J. et al. Recurrent Urinary Tract Infections: Unraveling the Complicated Environment of Uncomplicated rUTIs. Front. Cell. Infect. Microbiol. 11, 562525 (2021).
9. De Seta, F., Lowery, J. W., Oms, O. O., Ii & Larsen, B. Preclinical evaluation of pharmaceutical compositions and their components for urinary health. Open J. Obstet. Gynecol. 10, 151–165 (2020).
10. De Seta, F., Johnson, Z., Stabile, G., Martin, A. & Larsen, B. Rational development and evaluation of novel formulations for urinary health. Eur. J. Obstet. Gynecol. Reprod. Biol. 269, 90–97 (2022).
11. Bardella, M. T. et al. Gluten intolerance: gender- and age-related differences in symptoms. Scand. J. Gastroenterol. 40, 15–19 (2005).
12. Adults following flexitarian diet in Great Britain 2019-2021, by gender. Statista https://www.statista.com/statistics/1258008/adults-following-flexitarian-diet-in-great-britain-by-gender/.
13. Gorvett, Z. The mystery of why there are more women vegans. BBC.