Radiotherapy is one of the main treatment options for many different types of cancers as it has a high success rate in destroying cancerous cells. However, there is no existing way to prevent the inevitable harm caused by the ionizing radiations on neighbouring healthy cells and tissues.
60-80% of patients undergoing radiotherapy for abdominal or pelvic cancers displayed radiation-induced intestinal damage among other complications.1 Nicotinamide mononucleotide (NMN), the precursor of NAD+ (Nicotinamide Adenine Dinucleotide), has been shown to bring many benefits to its users and NMN supplementation is shown to relieve the aforementioned damage and complications caused by radiotherapy.
Radiation induced intestinal damage and complications
The intestinal tract is notable for the fast renewal and proliferation of its cells, which results in the organ’s increased receptivity to stimulation from the environment. Patients who undergo radiotherapy for the treatment of abdominal and pelvic tumours are at an increased risk of developing early intestinal injury manifestations such as abdominal distention, abdominal pain, nausea, loss of appetite, and acute diarrhoea.2 These symptoms have significant negative impact on the patient's quality of life. Additionally, older adults usually have an altered intestinal structure, decreased regenerative and proliferative ability, and other aspects, all of which could make their intestines more susceptible to ionized-radiation treatments.1,3
Chronic intestinal deterioration, a significant unfavourable consequence in long-term cancer survivors, typically occurs anywhere from a few months to three years after radiotherapy treatments and can result in microvascular sclerosis, intestinal fibrosis, and mucosal atrophy. This type of damage to the intestines can be life-threatening.2
1. Radiation Induced Intestinal Damages Mitigated by Modulating Gut Microbiota
A link between gut microbiota and radiation induced intestinal injury
Recent studies have shown that there is an irrefutable link between the gut microbiota and radiation-induced intestinal damage.1,3,4 These studies have demonstrated that radiation alters the gut microbiome in relation to its number and diversity. These alterations have affected the gastrointestinal system resulting in complications such as diarrhoea and enteritis.
Studies have indicated that ionising radiation (IR) has lead to a reduction in beneficial bacteria and an increment of harmful bacteria. It is suggested that this leads to higher intestinal sensitivity to inflammation, predisposing the gut mucosa to radiation-induced damage. The microbiota of the gut is closely related to side effects of radiotherapy.1
Furthermore, studies have shown that mice who were pre-treated with antibiotics (germ-free) were less affected from radiation therapy, insinuating that there is an undeniable relationship between gut microbiome and radiation-induced intestinal damages (Crawford and Gordon 2005; Zhao et al. 2022). 1,7 In addition, evidence suggests that treatment with probiotics and transplantation of microbiota from healthy mice to mice exposed to long-term radiation has alleviated radiation-induced acute intestinal injury.1
Uthever® NMN modulates gut microbiota and intestinal fibrosis
A study sponsored by Uthever® found that supplementation of Uthever® NMN reshaped the composition of gut microbiota and alleviated intestinal fibrosis caused by abdominal and pelvic cancer radiotherapy.1 Mice were employed in the study and were irradiated in the abdomen and then given NMN supplements. After irradiation, faeces were collected and 16S rRNA sequencing was done to identify the gastrointestinal tract microbiota.
It was found that supplementation of Uthever® NMN over a long period changed the composition and function of gut microbiota that had been disrupted by ionizing radiation (IR). NMN treatment resulted in increased abundance of beneficial bacteria and inhibited the proliferation of harmful bacteria.1 “The alterations in the composition of the gut microbiota eventually contribute to the changes in its functions, including cellular processes, metabolism, and human diseases,” Zhao et al. stated.1
The same study also detected the collagen deposition in colonic tissues using Masson’s trichrome staining (collagen stained in blue) and showed that ionizing radiation (IR) significantly increased collagen deposition leading to intestinal fibrosis. The study also demonstrated how NMN supplementation inhibited this process leading to reduced fibrosis.
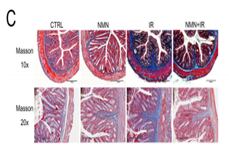
Above: The Masson staining's representative pictures (Source: Zhao et al., 2022)1
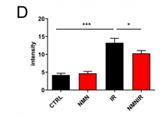
Above: A histogram displaying the intensity of the Masson stain (Source: Zhao et al., 2022)1
As there is an intimate relationship between the gut microbiota and radiation-induced intestinal injury, the alterations in microbiota brought about by Uthever® NMN were able to successfully counteract the effects of radiotherapy and help in preservation of beneficial effects of the gut microbiota, and thereby reduce the risks and relieve harmful effects of IR induced intestinal injury (Zhao et al. 2022).1
2. NRF2: Reducing Radiation-Induced Intestinal Damages by Strengthening Antioxidant and DNA Repair Mechanisms
Radiation and DNA damage: Role of NRF2
The principle behind radiotherapy for cancer cells is the destruction of cancer cells using ionizing radiation (IR). IR can induce production of Reactive Oxygen Species (ROS) or act directly on the genome to induce DNA damage in cancer cells. Ionizing radiation induces radiolysis of molecules of water, which results in the production of a number of free ions, electrons, and radicals, including the hydroxyl radical (-OH), increasing oxidative stress and further provoking DNA damage.5,6 These radiations can also damage neighbouring normal cells and tissues as well. As for abdominal and pelvic cancer radiotherapy, IR usually promotes intestinal injury and causes adverse effects. Elderly populations are affected more severely as their antioxidant and DNA repair systems weaken with aging.
A transcription factor called nuclear factor erythroid-2-related factor 2 (NRF2) binds to the antioxidant response element (ARE) in DNA and regulates expression of genes involved in DNA repair and antioxidant systems. Therefore, NRF2 inducers have been used as powerful antioxidant therapeutic drug in order to cure ailments that are brought on by radiotherapy of cancerous tissues/cells.8,9
Sirtuins are a class of proteins which are known to be closely associated with aging and longevity. They also involved in supporting antioxidant and DNA repair systems. For example, SIRT1, SIRT6 and SIRT7 are the three members of Sirtuin family found in the nucleus, which might be able to regulate the expression of antioxidant genes by interacting with NRF2. SIRT1, SIRT6, and SIRT7 have been found to have a role during DNA damage response.
Uthever® NMN helps elevate NRF2
Another study sponsored by Uthever® found a promising solution for alleviating IR-induced damages by elevating the NRF2 levels with the help of NMN supplementation. NMN has an agonistic effect on NRF2. The study which was conducted on mice to evaluate the effects of Uthever® NMN on alleviating intestinal injuries caused by radiotherapy of cancer treatments, found that lowering or silencing the level of NRF2 enhances reactive oxygen species (ROS) levels and makes body tissues more vulnerable to radiation, which ultimately increases the severity of IR-induced side effects (Zhao et al., 2022).6 Furthermore, it was proven that Uthever® NMN administration, could reverse the damages and offer a shielding effect by repairing DNA damage and alleviating intestine injury caused by cancer radiotherapy.
Some of NAD+ dependent sirtuins family members (SIRT1-7 i.e. SIRT1, 2, 3, etc) that involve in longevity also act alongside NRF2. NMN also have a synergistic effect on these proteins. Uthever® NMN administration was found to have enhanced the effects on these proteins as well (Zhao et al., 2022).6 It was found that the excess of SIRT7 and SIRT6, as well as the NMN administration, could reverse the damages and offer a shielding effect by repairing DNA damage and alleviating intestine injury caused by cancer radiotherapy.
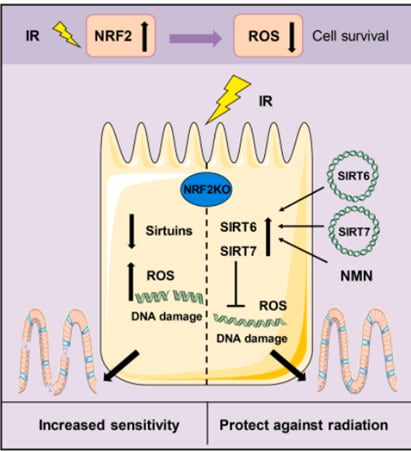
Above: NMN attenuated IR-reduced NDA damage and intestine injury (Source Zhao et al., 2022)6
SIRT6/SIRT7 expression by NMN supplementation reduced IR-induced DNA damage and intestinal injury in the aged NRF2 deficient animal.
In conclusion, studies have shown that NMN supplementation could succeed in counteracting the negative impact of radiotherapy and thereby enhance its potential as a viable and effective treatment for cancer. Clinical studies are called for to further verify that the same benefits of NMN supplementation applies to human cancer patients.
NMN may very well be the key to unlock many of the present-day health related advancements. Its approach in relation to other spheres of health such as skin treatment, anti-aging effects and infections, will be the focus of future scientific studies.
References
1. Zhao, X.; Ji, K., Zhang, M.; Huang, H.; et al (2022). NMN alleviates radiation-induced intestinal fibrosis by modulating gut microbiota. International Journal of Radiation Biology, 1-12.
2. Hauer-Jensen, M., Denham, J. W., & Andreyev, H. J. N. (2014). Radiation enteropathy—pathogenesis, treatment and prevention. Nature reviews Gastroenterology & hepatology, 11(8), 470-479.
3. Martin, K., Potten, C. S., Roberts, S. A., & Kirkwood, T. B. (1998). Altered stem cell regeneration in irradiated intestinal crypts of senescent mice. Journal of cell science, 111(16), 2297-2303.
4. Nam YD, Kim HJ, Seo JG, Kang SW, Bae JW. 2013. Impact of pelvic radiotherapy on gut microbiota of gynecological cancer patients revealed by massive pyrosequencing. PLoS One. 8(12):e82659
5. Bai, J., Barandouzi, Z. A., Rowcliffe, C., Meador, R., Tsementzi, D., & Bruner, D. W. (2021). Gut microbiome and its associations with acute and chronic gastrointestinal toxicities in cancer patients with pelvic radiation therapy: a systematic review. Frontiers in oncology, 11, 5237.
6. Zhao, X., Zhang, M., Wang, J., Ji, K., Wang, Y., Sun, X., & Liu, Q. (2022). NMN ameliorated radiation induced damage in NRF2-deficient cell and mice via regulating SIRT6 and SIRT7. Free Radical Biology and Medicine, 193, 342-353.
7. Crawford PA, Gordon JI. 2005. Microbial regulation of intestinal radiosensitivity. Proc Natl Acad Sci U S A. 102(37):13254–13259
8. Wei, J. et al. (2021) “Sulforaphane-mediated Nrf2 activation prevents radiation-induced skin injury through inhibiting the oxidative-stress-activated DNA damage and NLRP3 inflammasome,” Antioxidants (Basel, Switzerland), 10(11), p. 1850. doi: 10.3390/antiox10111850.
9. Nakagami, Y. (2017) “Nrf2 activators as therapy for acute radiation dermatitis,” Journal of Rare Diseases Research & Treatment, 2(3), pp. 11–15. Doi: 10.29245/2572-9411/2017/3.1109.








