Flore Larger, corporate communications manager for the French pharma and nutrition firm, told NutraIngredients that it would be contesting the opinion which rejected its request to broaden the approved claim to include powdered supplements diluted in water at 2 g per day, but declined to specify how it plans to do this. “We will make every effort to contest this negative opinion as this authority recognized evidence of effect of our study for this specific claim,” she said.
Larger said the application was an attempt to gain a claim specific to its product, Novasterol, but that the recent rejection would not have an impact on its ability to market its supplement since it can continue to market the supplement under an existing general claim.
Commercialisation not at stake

“We filed to have a specific claim on Novasterol. But commercialisation is not at stake,” she said. “It is already on the market and will continue to be.”
“This opinion has no impact on Novasterol commercialisation as it is based on the general claim for phytosterol stating that they have positive effects on lowering LDL blood level,” she said.
Contesting the claim
The firm maintains that the unpublished study, which used its product, quantifies the efficacy of phytosterols in powder on LDL blood levels within 6 weeks.
However, in its decision, EFSA’s Panel on Dietetic Products, Nutrition and Allergies (NDA), said: “In weighing the evidence, the Panel took into account that only one human intervention study showed a reduction in blood LDL-cholesterol concentrations after six weeks of consuming 2 g/day of plant sterol esters in powder, the large uncertainty surrounding the estimates of this effect, and that the results of that study have not been replicated in other studies.”
Instead the panel said it wished to reiterate its previous conclusion that, while plant sterols added to foods like margarine-type spreads, mayonnaise, salad dressings, milk, yoghurts and cheese have been shown “consistently to lower blood LDL-c concentrations in a large number of studies”, the effective dose of plant sterols as powder diluted in water needed to achieve this effect in a particular timeframe has yet to be established.
Larger said the company was currently in the process of preparing its contestation dossier, but declined to give more details on this.
Sanofi-Aventis France
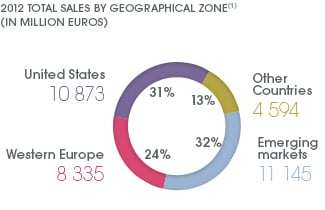
According the company its net global sales stood at €34,947m in 2012. 32% of this was derived from emerging markets like China, India, Brazil and African countries, followed by the US (31%), Western Europe (24%) and other countries like Japan, Canada, Australia and New Zealand (13%).
Outside of nutraceuticals the company works within vaccination, insulin and animal health.
Last year the company was ordered to pay €28m in fines after German courts found that two of its employees had made illicit payments to a consultancy advising one of its clients, giving the firm unfair preference.
