EC follows EFSA opinion and lowers vit D levels in infant formula

Document updates published in the Official Journal of the European Union, now order infant formula to contain a minimum vitamin D content of 0.48 micrograms (μg) per 100 kilojoules (/kJ) or 2μg per 100 kilocalories (kcal).
The maximum amounts now stand at 0.6 micrograms (μg) per 100 kilojoules (/kJ) or 2.5μg per 100 kcal.
Meanwhile erucic acid requirements for infant formula and follow-on formula now state, “The erucic acid content shall not exceed 0.4% of the total fat content.”
EFSA judgement
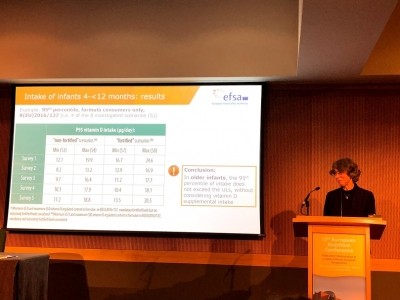
The changes are made on the advice of the European Food and Safety Authority’s (EFSA) opinion in 2018 that highlights the possible risk of infants exceeding vitamin D upper limits when consuming formula.
“In its Scientific Opinion of 28 June 2018, the Authority concluded that the use of infant formula containing vitamin D at 3 μg/100 kcal may lead some infants aged up to 4 months to consume amounts of vitamin D above the tolerable upper intake level from the formula alone,” the EC stated.
“That opinion also concluded that the use of a maximum vitamin D content of 2,5 μg/100 kcal in infant formula does not result in intakes of vitamin D above the tolerable upper intake level from the formula alone,” the Commission added.
“On the basis of that opinion, the maximum vitamin D content permitted under Delegated Regulation (EU) 2016/127 for infant formula should be lowered to 2,5 μg/100 kcal, in accordance with Article 6 and paragraphs (1) to (4) of Article 9 of Regulation (EU) No 609/2013.”
Erucic acid considerations
Meanwhile, there were minor concerns involving erucic acid content in infant formula and follow-on formula.
EFSA adopted a scientific opinion on the presence of erucic acid in feed and food, which concluded that the 95th percentile dietary exposure level was highest in infants and other children
The Authority thought this may indicate a risk for young individuals with high erucic acid exposure.
“Taking into account the conclusions of the opinion, it is appropriate to lower the maximum levels of erucic acid in infant formula and follow-on formula,” the EC said.