This latest request arises in the context of Commission Delegated Regulation (EU) 2016/1273, which will repeal Directive 2006/141/EC4, effective from 2020.
In describing the methodology used in the update, Monika Neuhäuser-Berthold, gave a unique insight into how the Authority came to its conclusions despite a “paucity of data on infants on which to base a no‐ or a lowest observed adverse effect level (NOAEL or LOAEL)”.
Speaking at The Federation of European Nutrition Societies (FENS) conference, Neuhäuser-Berthold, began by identifying the target population of full term, healthy infants below the age of one year.
“Intake assessment of vitamin D was also accompanied by a systematic literature review consisting of 31 studies from 25 papers,” said Neuhäuser-Berthold, the chair of the European Food Safety Authority (EFSA) working group on Dietary Reference Values for vitamins.
“These studies included trials and observational studies that used full-term healthy infants, breastfed or formula-fed, who may have received complementary foods.
“The studies showed inconsistent results or the absence of a dose-response relationship between vitamin D and the adverse health outcomes (hypercalciuria, hypercalcaemia, nephrocalcinosis and growth).
“We concluded that data on daily vitamin D intake and these outcomes could not be used alone to set a UL for vitamin D.”
Unlikely risk
Further analysis of the dose-response found that the estimated % of infants with a level of 200 nanomols per litre (nmol/L) of the major circulating form of vitamin D, 25-hydroxyvitamin D (25(OH)D) was “unlikely to pose a risk of adverse health outcomes in healthy infants.”
This value was derived from previous assessments (EFSA & other bodies) and from data on infants from the systematic review.
Neuhäuser-Berthold pointed out that this value was not a cut-off for toxicity but a conservative value from which a UL could be derived.
Further statistical models used for this update indicated that the percentage of infants exceeding a S25(OH)D level of 200 nmol/L appeared to increase with supplemental vitamin D intakes or with baseline serum 25(OH)D concentrations. This percentage also looked to have decreased with age.
Conclusion reached on these updated ULs identified that up to 6 months, the available body of evidence supported keeping the previous UL of 25 micrograms per day (µg/day).
Further recommendations concluded that for infants six to less than 12 months, evidence from the predictions supported a UL of 35 µg/day, which according to Neuhäuser-Berthold, may be explained by the increase in body mass.
Infants upto 4 months
The intake assessment for infants up to 4 months took on a slightly modified direction with the assessment based on calculations in exclusively formula-fed infants.
This included daily infant formula daily consumption, body weights, and the vitamin D content contained in the infant formula.
The calculations for the estimated “high” intake of vitamin D factored in infant formula only at one and four months.
“The calculations were based on the maximum regulatory vitamin content in the infant formula and the default level set for high infant formula consumption as dictated by EFSA’s scientific committee in 2017,” said Neuhäuser-Berthold.
“Median reference body weights taken from the World Health Organisation (WHO) standards were also used for this calculation.”
Results for the intake of infants up to 4 months concluded that increased levels of vitamin D in infant formula to 3 µg/100 kcal may lead some younger infants to consume amounts above the UL from formulae alone (25 µg/day).
The conclusion also stated that the precise percentage of infants could not be determined from the data used.
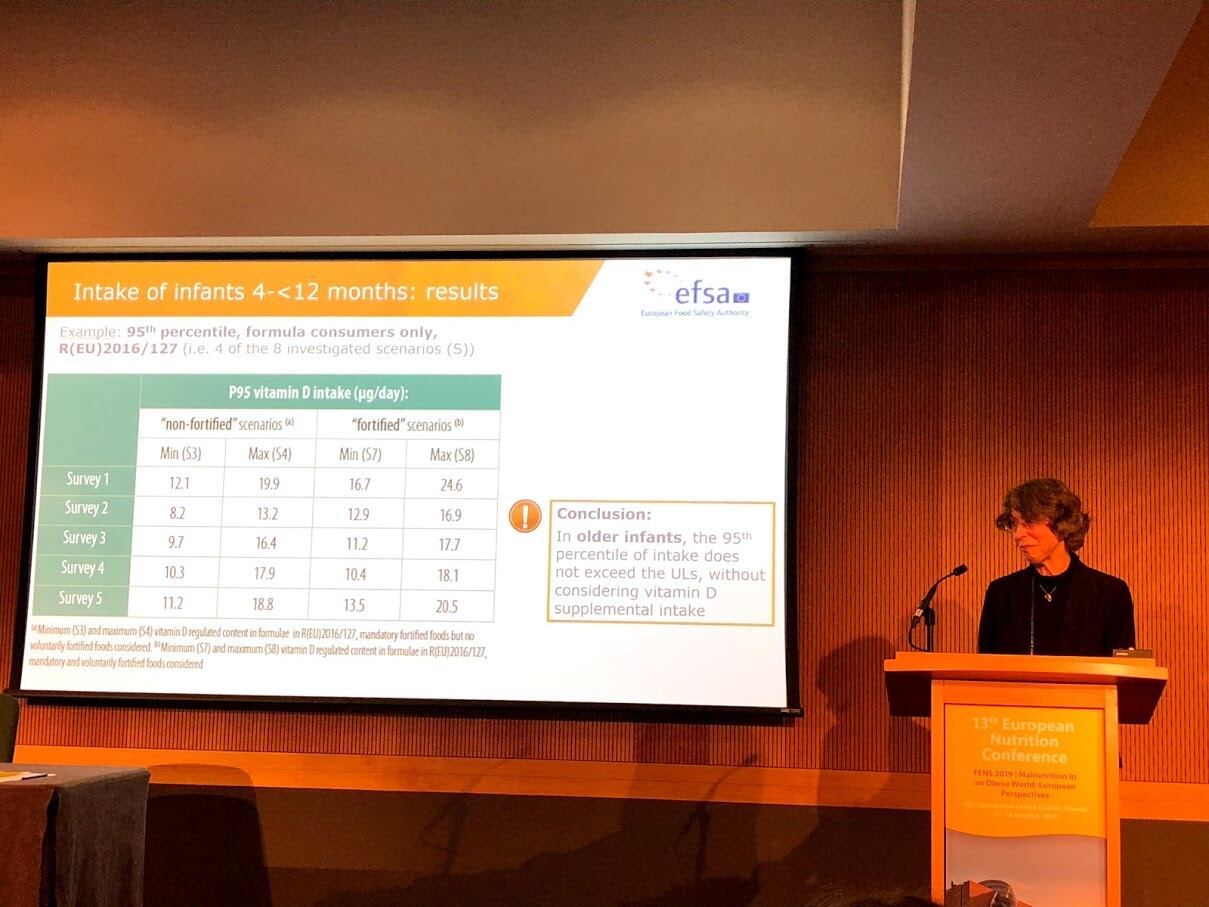
Infants between 4 - 12 months
Neuhäuser-Berthold also revealed the criteria used for assessing the intake of vitamin D in infants between four and 12 months of age.
Here, individual food consumption data from EFSA’s European Comprehensive Consumption Database was used including foods naturally containing vitamin D. EFSA’s nutrient composition databased was also used to assess vitamin D food content.
Additionally, foods voluntarily fortified with vitamin D and foods mandatorily fortified with vitamin D (infant formula, follow-on formula, processed cereal-based foods for infants and young children) were also used.
EFSA concluded that for older infants, the 95th percentile of intake does not exceed the ULs, without considering vitamin D supplemental uptake.