Recall round-up: Vitamin A, B17 and red rice supplements

Health authorities in Spain have used the European Food Alert Network (RASFF), to alert countries of the presence of unlabelled soy in the food supplement, Cholkur Advance.
The product, a supplement containing Monacolin K extracted from red rice, is said to help maintain healthy blood cholesterol levels and promote weight loss.
The lot in question ‘0599’ is available in packs of 30 tablets (550 mg) with a best before date of 09/30/2023.
“The Spanish Agency for Food Safety and Nutrition (AESAN) advises consumers with an allergy to soy that the marketing of the Cholkur Advance food supplement contains soy not included in the labelling.
“As a precautionary measure, those consumers with an allergy to soy who may have the aforementioned product in their homes are recommended to refrain from consuming it. The consumption of this product does not pose a risk for other consumers.”
Staying with AESAN, the agency has notified consumers of the withdrawal of Vicanplus capsules, a food supplement found to contain sildenafil and tadalafil, not declared on its labelling
Lab analysis found quantities of sildenafil and tadalafil were sufficient enough to ‘restore, correct or modify a physiological function, exerting a pharmacological action.’
“For these reasons, the Spanish Agency for Medicines and Health Products (AEMPS) has ordered a ban on the marketing and withdrawal from the market of all copies of the aforementioned product.”
The product Vicanplus capsules are distributed by Natural Innova and are available in packs of 4 capsules
High amygdalin content
The Czech Agriculture and Food Inspection Authority (CAFIA) has identified ProfiVit Vitamin B17 supplements as containing a high content of amygdalin.
The product, available in packs of 210 capsules, is produced by the Slovakian firm ProFivit s.r.o., where CAFIA officials took the sample from the Czech-based website ‘https://enerex-vitaminy.cz.’
Officials explained that amygdaline can metabolise to hydrogen cyanide and cyanides within the human body.
Lab analysis confirmed the presence of amygdalin amounting to 493 milligrams (mg) in one recommended daily dosage, which corresponds up to 29mg of hydrogen cyanide after metabolising.
“The presence of amygdalin is not a case of coincidental contamination, but intentionally added substance,” CAFIA officials stated.
The authority has ordered the withdrawal of all batches of the product from the market and has strongly warned all consumers against consumption of the food supplement.
Faulty vitamin A batches
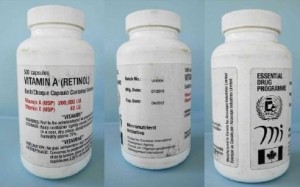
Finally, the World Health Organisation (WHO) issued a product alert earlier in the month warning of two batches of vitamin A that were deemed as ‘severely degraded and underdosed.’
The batches, which were supplied to patients in Chad, were found to have carried now-defunct logos, specifically the WHO Essential Drugs Programme logo and the Micronutrient Initiative logo.
WHO officials confirmed the products as falsified due to the variable data (batch number and expiry dates) of these products that did not correspond to genuine manufacturing records.
Of more concern was the discovery of the product’s manufacturing and expiry dates that had been deliberately altered to extend their shelf-life.
Genuine Vitamin A (Retinol), batch UI4004, was manufactured by Accucaps Industries in September 2009, with an expiry date of September 2012. Lab analysis found the capsules contained only 68.6% of the stated active ingredient.
Genuine Vitamin A (Retinol), batch 39090439, was manufactured by Banner Pharmacaps (Canada), with an expiry in 2009. Lab analysis found the capsules contained only 64.4% of the stated active ingredient.
The WHO were keen to stress that Accucaps Industries and Banner Pharmacaps were in no way responsible for the falsification and were not the subject of its investigations.
“All medical products must be obtained from authorized/licensed suppliers,” the WHO said. “The products’ authenticity and physical condition should be carefully checked. Seek advice from a healthcare professional in case of doubt.
“If you are in possession of the above falsified products, please do not use them.”