EHPM: ‘Plant claims regulation must prioritise choice, consumers and harmonisation.’

Speaking to NutraIngredients, the European Federation of Associations of Health Product Manufacturers (EHPM) argues that placing these aims top of the agenda would “provide the basis for a more balanced regulation that addresses the needs and aspirations of consumers alongside those of regulators and the industry.”
Responding to findings that think the objectives of the Claims Regulation have not been fully attained, the EHPM points out the actual objectives stated in the Articles of the Health Claims Regulation, refer to protecting consumers from being misled and to harmonising the market.
“However, the preamble of the Regulation also focuses on the importance of providing consumers with the information they need to make informed choice,” the group says.
“The omission of this aspiration from the objectives has led to a regulation that is excessively restrictive.”
The European Commission’s (EC) staff working document attempts to make sense of a recent EC study evaluating Regulation (EC) No 1924/2006 on nutrition and health claims that calls for clarification of 'traditional use' in efficacy assessments of health claims on plants used in foods.
This action extends to possible implications the coexistence of Traditional Herbal Medicinal Products (THMP) on the same plant substances may have on the EU market.
“In the light of these shortcomings about the smooth functioning of the internal market and the possible openness to the notion of 'traditional use' to substantiate health claims on plants, there are merits for further studying the potential EU harmonisation of the field of plants, including the safety aspect,” the document states.
Botanicals in food supplements
The EHPM, a Brussels-based association representing health product manufacturers in Europe, acknowledges that while the EC Document focuses on health claims, references are also made to safety concerns regarding botanicals used in food supplements.
The EC identifies Art. 8 of Reg. 1925/2006 as the legislative tool to be used to establish a list of plants and other ingredients prohibited for use in food supplements, arguing that its timelines are extremely stringent for the industry and disproportionate for the consumer.
“In the absence of a dialogue with the European Food and Safety Authority (EFSA), and of an appropriate safety assessment method for botanical substances, it is extremely challenging for companies to have clarity on the type of data they will need to submit to substantiate the safety of the ingredient under assessment,” the EHPM says.
“This process is therefore unsustainable for the food supplement sector in the long term.”
Concerns have been heightened by the ongoing coronavirus situation, in which the EHPM points to the circulation of untrue or misleading information set out to influence consumer purchasing choice.
“It would be useful to have claims available to inform consumers on how certain botanicals function to support the immune and respiratory health and so allow them to differentiate these from those that do not. But to link this process to a COVID-19 benefit is a step too far,” the Association says.
“As EHPM we strongly support and embrace the campaign against misleading and false information, and we firmly state that food supplements do not cure, treat or prevent the coronavirus.”
EHPM action on claims
The EHPM are currently working on an alternative approach to botanicals claims, with the Association believing methodologies developed and applied by EFSA to be, ‘not so effective for claims associated with more complex foodstuffs (e.g. those obtained from plants and other vegetative organisms – ‘botanicals’),’ resulting in the ‘on-hold’ status.
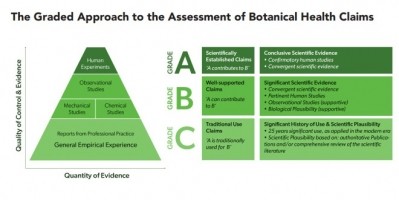
In its Manifesto, the EHPM Working Group on Botanicals, propose three grades of claim assessment, each supported by appropriate levels of evidence: scientifically accepted claims, claims that are scientifically well-supported, and ‘traditional use’ claims.
The Group also stress that wording of the claims would need to be carried out in such a way that consumers would have access to clear and easy-to-understand information.
The different types of evidence required to substantiate claims would be identified and the level of evidence required for each type of claim is specified.
Botanical substances must be appropriately characterised; stringent scientific evaluation must be applied; and the honesty, accuracy and meaningfulness of claims must be assured.
“This approach has a number of important benefits,” the EHPM says. “It resolves the legal anomaly with the treatment of THMPs while benefiting from the legal robustness of the THMP approach.
“It also provides an incentive to SMEs that predominate in the food sector, and especially in the food supplement sector, encouraging businesses to engage in R&D by providing a practical solution they can benefit from;
“It also provides consumers with controlled information that they are looking for on the food, and in particular the food supplements, that they purchase and consume.
“Of course, this is the EHPM proactive proposal and we are looking forward to exchanging with the EU and National Institutions as well as with stakeholders to find a solution that address everyone’s needs and will meet the objectives of the Claims regulation.”