Aging is an inevitable process of life, and for women, ovarian health and the onset of menopause are particularly important topics.
In April 2025, a research team involving scientists from Sorbonne University expanded the 12 hallmarks of aging to 14, offering a more comprehensive understanding of the mechanisms underlying aging.¹ These hallmarks are a key way to explore their connection with premature ovarian insufficiency (POI) and female menopause.
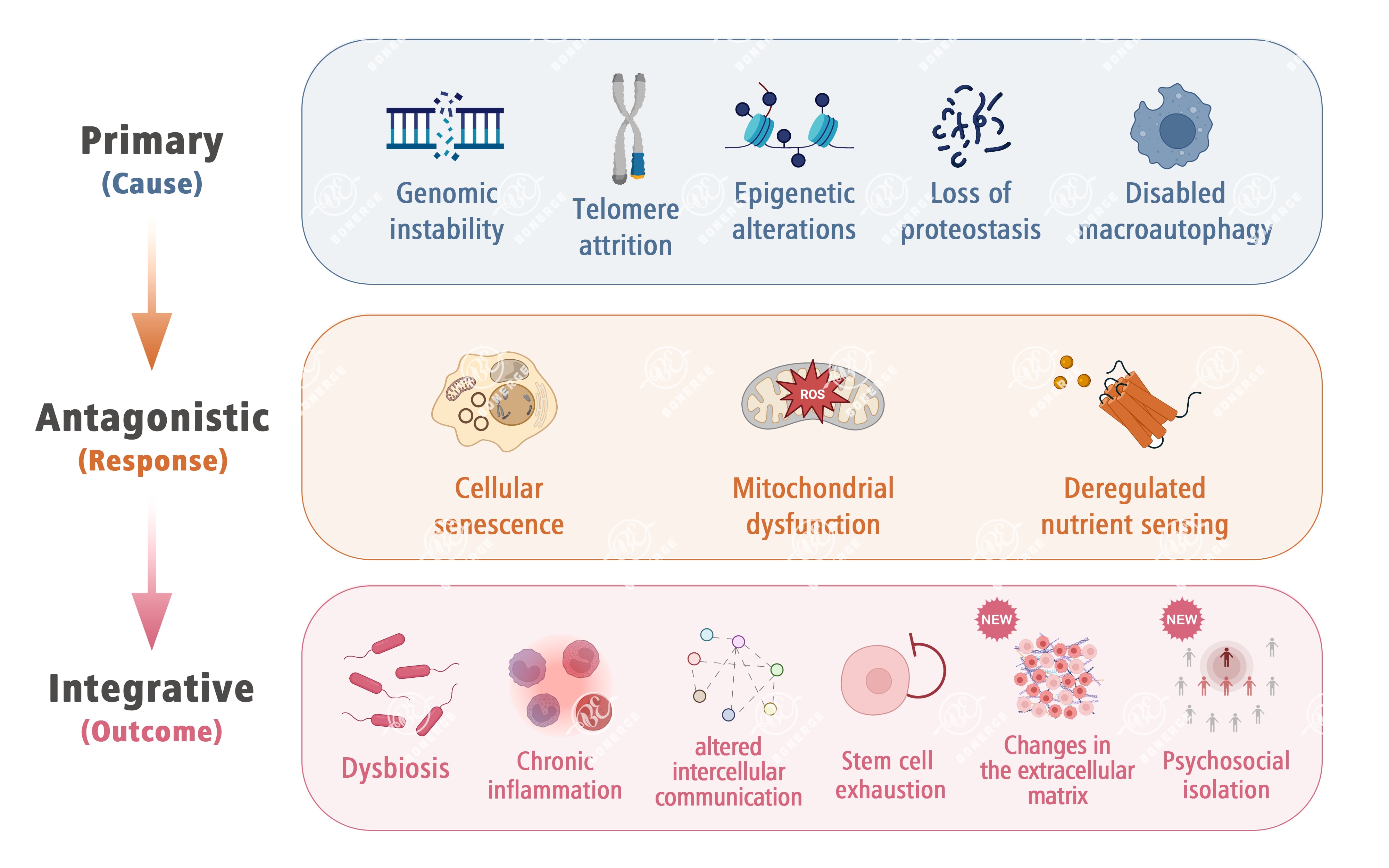
First: what are the 14 hallmarks of aging? The 14 hallmarks of aging are not randomly listed; they are logically categorized into three groups based on a ‘cause’, ‘response’, ‘outcome’ framework, together forming a comprehensive ‘panorama’ of aging:¹⁻²
Primary hallmarks: The ‘root drivers’ of aging These include genomic instability, telomere attrition, epigenetic alterations, loss of proteostasis, and disabled macroautophagy. These are the fundamental drivers of aging, analogous to core component failures in a machine, which directly trigger the aging process.
Antagonistic hallmarks: The ‘stress responses and side effects’ of aging These include cellular senescence, mitochondrial dysfunction, and deregulated nutrient sensing. They represent the body’s adaptive responses to primary hallmarks. Although they are initially protective, their prolonged activation or dysfunction may accelerate aging.
Integrative hallmarks: The ‘final observable manifestations’ of aging These include dysbiosis, chronic inflammation, altered intercellular communication, stem cell exhaustion, changes in the extracellular matrix (new), and psychosocial isolation (new). These result from the long-term effects of primary and antagonistic hallmarks. They directly correspond to observable aging phenotypes and chronic disease risks.
Focus on ovarian health: Antagonistic hallmarks as the ‘key breakthrough’
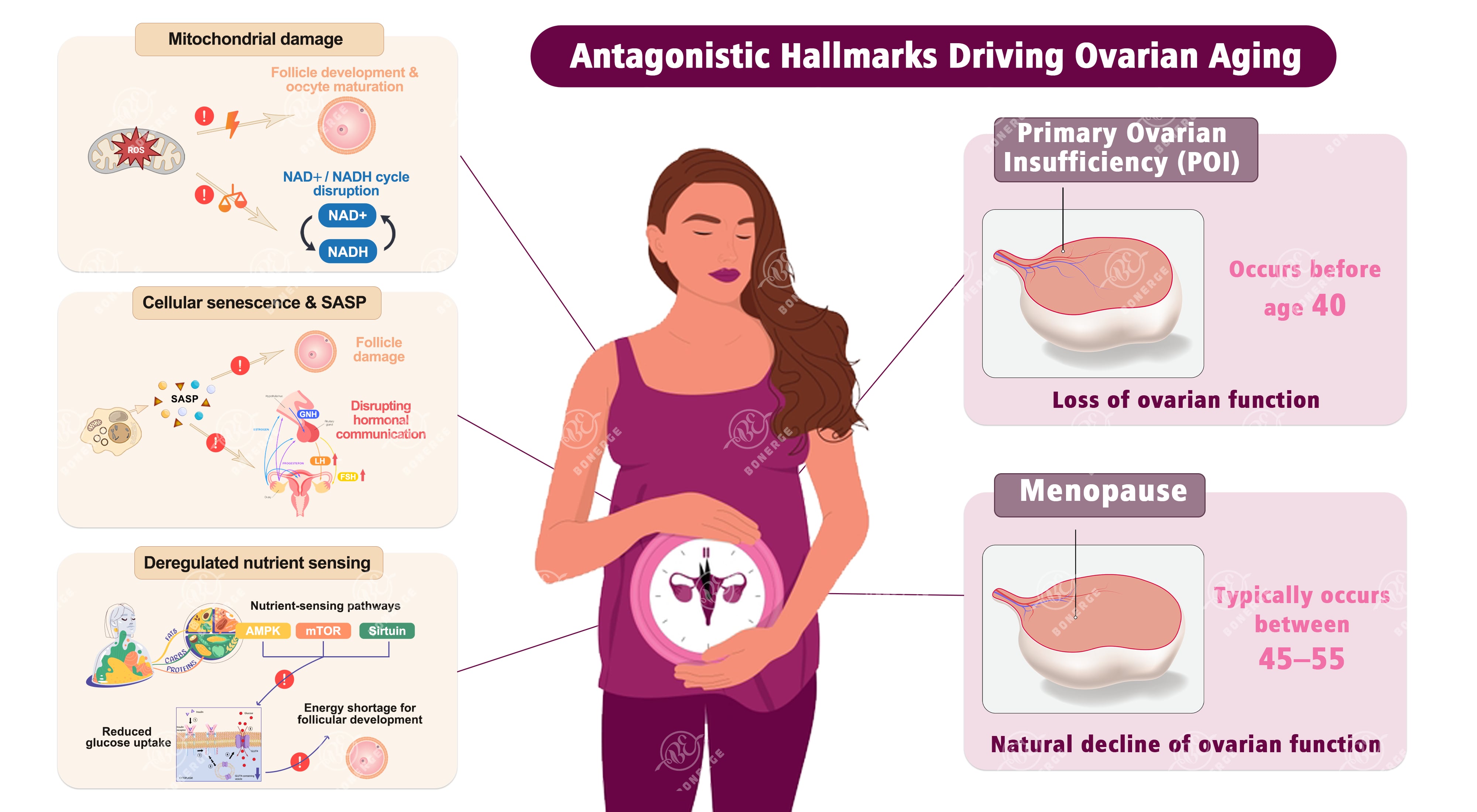
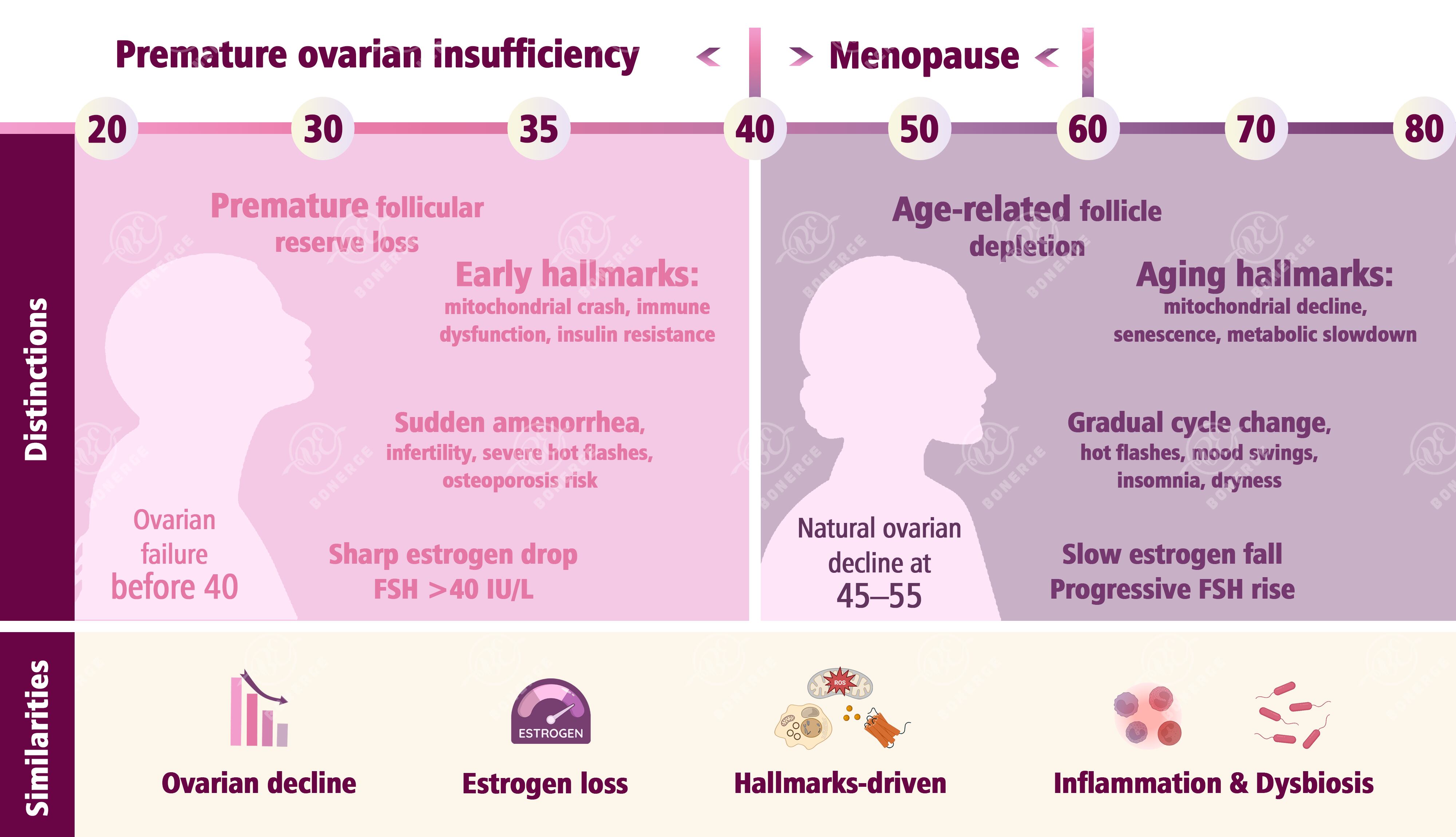
The ovaries are central to female endocrine and reproductive function, and their health is closely linked to the aging process. Particularly in POI (defined as the loss of ovarian function before the age of 40) and menopause (typically occurring between 45 and 55 years of age due to the natural decline of ovarian function), the antagonistic hallmarks in aging biology offer a critical explanatory framework and potential intervention targets.³
POI: Antagonistic hallmarks ‘spinning out of control early’
POI is essentially the premature activation of aging programs in ovarian cells, with abnormal antagonistic hallmarks playing a major role:
Mitochondrial damage: An energy crisis in ovarian cells
The development of follicles and the maturation of oocytes require a substantial amount of energy, which is mainly supplied by mitochondria. Oocytes contain the highest number of mitochondria among human cells, highlighting their high energy dependence.
When mitochondria become dysfunctional due to oxidative damage, genetic mutations, or other factors, follicular cells experience an energy deficit – failing to complete normal cell division and differentiation, and may undergo premature apoptosis. Mitochondrial dysfunction also disrupts the NAD+/NADH cycle, further worsening metabolic imbalance and ultimately leads to rapid follicular depletion and POI.⁴
Cellular senescence and SASP: Chronic pollution in ovarian tissue
Immune cells (e.g. macrophages and T cells) in the ovaries are responsible for clearing damaged follicles and senescent cells. However, as individuals age or are exposed to external stressors (e.g. psychological stress, environmental pollution), immune cells themselves undergo senescence and lose their ability to clear damaged cells.
Accumulated senescent cells release SASP (senescence-associated secretory phenotype) – inflammatory factors (e.g. IL-6, TNF-α) that damage healthy follicles, and proteases that degrade the ovarian extracellular matrix. Analogous to planting time bombs in the ovary, these senescent cells continuously disrupt the ovarian microenvironment and accelerate the decline of ovarian function.⁵
Deregulated nutrient sensing: Systemic hyperglycemia and insulin resistance
Nutrient-sensing pathways (mainly AMPK, mTOR, and sirtuin) are key signaling switches regulating cellular metabolism, aging, and reproductive function. Dysregulation of these pathways reduces cellular sensitivity to nutrient signals, inhibits insulin-mediated translocation of the glucose transporter GLUT4 to the cell membrane, and decreases glucose uptake.
Consequently, even when blood glucose levels are sufficient, ovarian cells cannot utilize it effectively for metabolism, resulting in intracellular hypoglycemia and extracellular hyperglycemia. This directly affects the energy supply required for follicular development and further impairs insulin signaling.⁶
Female menopause: Antagonistic hallmarks naturally accumulate
Menopause results from the natural decline of ovarian function. It is essentially the outcome of long-term primary hallmark effects leading to comprehensive imbalance in antagonistic hallmarks.
Mitochondrial functional decline: From failure to natural aging
After the age of 40, the mitochondrial self-repair capacity gradually declines. Accumulated DNA mutations and decreased activity of antioxidant enzymes lead to a gradual decline in energy production – not a sudden failure but a gradual reduction.
This gradual energy deficit results in slower follicular development, reduced oocyte quality, decreased fertilization potential, and impaired function of granulosa cells (the primary estrogen-secreting cells), which leads to a gradual decline in estrogen levels and lays the groundwork for menopause.⁷
Cellular senescence accumulation: From local pollution to systemic imbalance
After the onset of menopause, there is a significant accumulation of senescent cells, and the damaging effects of SASP spread from local to systemic levels – not only damaging follicles but also disrupting hormonal communication among the ovaries, hypothalamus, and pituitary gland (e.g. reduced negative feedback of estrogen on the hypothalamus).
This leads to abnormal increases in gonadotropins (follicle-stimulating hormone, FSH and luteinizing hormone, LH), which causes menstrual irregularities, hot flashes, night sweats, and other menopausal symptoms.⁵
Deregulated nutrient sensing: From dysfunction to metabolic adaptation
In menopausal women, deregulated nutrient sensing is often a combination of age-related metabolic slowdown and hormonal changes. Such dysregulation (e.g. decreased sirtuin activity) accelerates the senescence of granulosa cells, shortens the survival period of follicles, depletes the follicular reserve at an earlier stage, and may result in earlier onset of menopause.
Meanwhile, imbalance in these pathways exacerbates metabolic disorders: estrogen decline already increases the mood swing to fat accumulation, and dysregulation of these pathways further inhibits AMPK-mediated lipolysis while promoting mTOR-driven lipogenesis. This not only increases insulin resistance risk but also amplifies menopausal symptoms such as hot flashes and osteoporosis.⁶
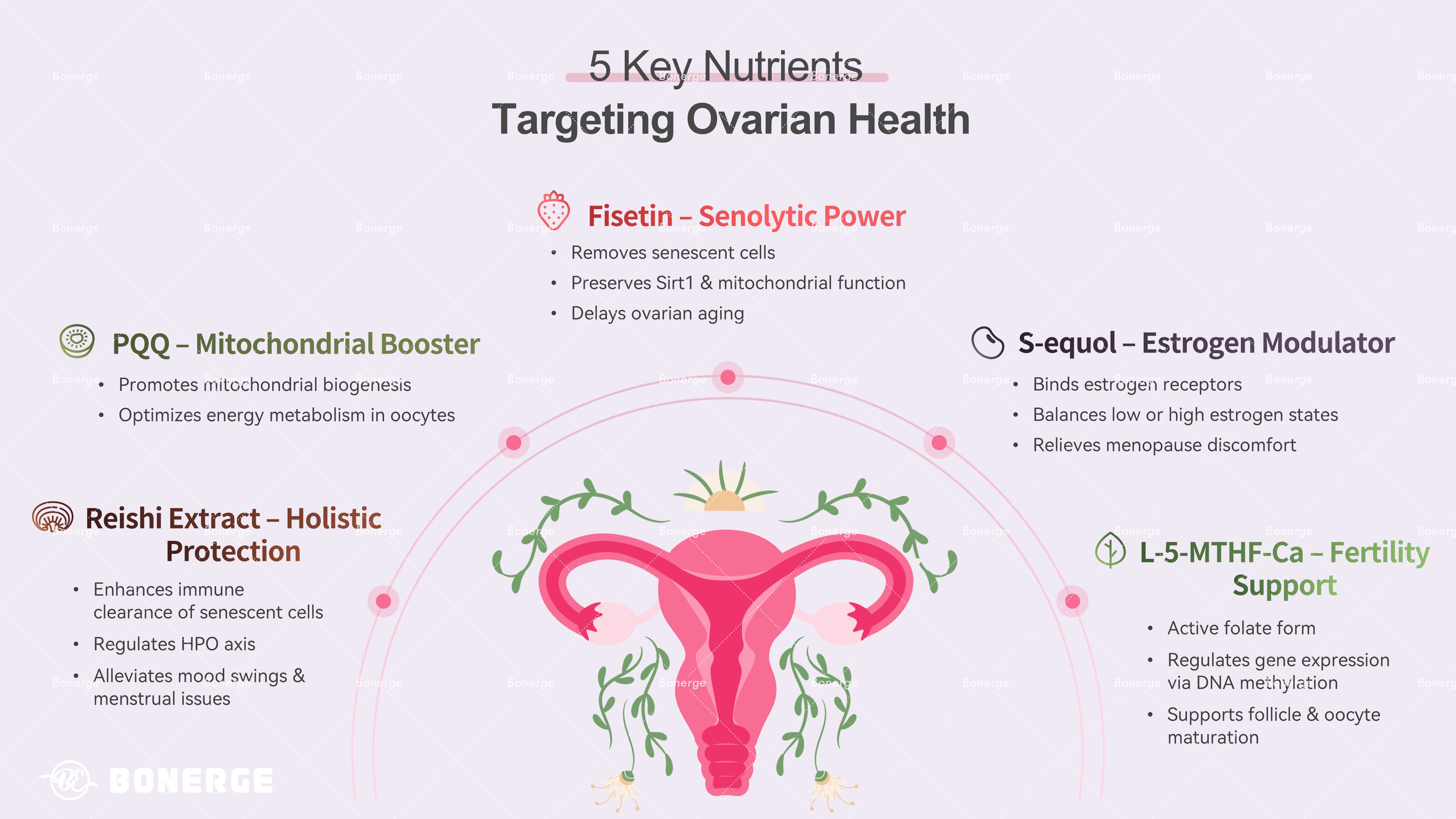
Protecting ovarian health: Five anti-aging targeting nutrients
Based on the core mechanisms of POI and menopause, scientific research has identified the following five nutrients that support ovarian health.
1. Fisetin: Natural senolytic clears senescent cells
Fisetin is a natural flavonoid present in strawberries and apples. It is recognized as the most potent natural senolytic compound that selectively remove senescent cells and induce their apoptosis, thereby reducing the accumulation of senescent cells in the ovary and minimizing the damage caused by SASP.
It also prevents the decline of Sirt1 – a key anti-aging protein – in aging oocytes, enhances mitochondrial function, restores the antioxidant capacity of the ovary, and improves energy metabolism, thus delaying ovarian aging.⁸
2. Pyrroloquinoline quinone (PQQ): Promotes mitochondrial biogenesis
PQQ is widely used in mitochondrial support formulations due to its ability to significantly enhance mitochondrial biogenesis, increase the number of healthy mitochondria within cells, and optimize cellular energy metabolism. This ensures an adequate energy supply for oocytes.⁹
3. S-equol: Selectively modulates estrogen
S-equol is an active metabolite of soy isoflavones produced by gut bacteria. However, due to individual gut microbiota variations, only 30% to 50% of individuals can convert soy isoflavones into S-equol to an effective level. Its structure is similar to human estrogen, allowing it to mildly and selectively modulate estrogen levels.
When estrogen levels are low (e.g. during menopause or POI), it binds to estrogen receptors and exerts weak estrogenic effects, thereby alleviating menopause discomfort like hot flashes and night sweats. When estrogen level is high, it competitively binds receptors, preventing overstimulation of the ovaries.¹⁰
4. L-5-Methyltetrahydrofolate Calcium: Improve fertility
L-5-Methyltetrahydrofolate Calcium (L-5-MTHF-Ca) is the bioactive form of folate that can be directly utilized by the human body. As a core carrier of methyl donors, L-5-MTHF-Ca regulates the expression of key genes in ovarian cells by participating in DNA methylation and histone modification. This process creates a stable epigenetic environment essential for follicular development and oocyte maturation. Due to these critical roles, L-5-MTHF-Ca is widely used to support and improve fertility.¹¹
5. Reishi mushroom extract: Comprehensive ovarian protection
Reishi mushroom extract (active components: polysaccharides and triterpenes) has long been regarded as a tonic in traditional medicine. Modern research confirms its multiple protective effects on ovarian health, including enhancing the activity of immune cells (e.g. macrophages and natural killer cells) to improve the efficiency of clearing senescent cells in the ovary and reduce the accumulation of SASP.
It also helps regulate the hypothalamic-pituitary-ovarian axis, inhibit abnormal increases in gonadotropins, and alleviate mood swings and menstrual irregularities associated with menopause.¹²
Ovarian health is central to female aging. The 14 hallmarks of aging provide a scientific framework for preventing POI and managing menopause. In addition to rational nutrient supplementation, maintaining regular sleep, following a balanced diet, engaging in moderate exercise, and practicing stress management are equally important.
While aging is inevitable, decline is not. With scientific strategies, women can be supported to proactively slow ovarian aging and boost their wellbeing, improving the quality of life.
References
- Kroemer, G.; et al. From Geroscience to Precision Geromedicine: Understanding and Managing Aging. Cell. 2025 Apr 17;188(8):2043–2062.
- López-Otín, C.; et al. Hallmarks of Aging: An Expanding Universe. Cell. 2023 Jan 19;186(2):243–278.
- Wu, C.; et al. Hallmarks of Ovarian Aging. Trends Endocrinol Metab. 2025 May;36(5):418–439.
- Chiang, JL.; et al. Mitochondria in Ovarian Aging and Reproductive Longevity. Ageing Res Rev. 2020 Nov;63:101168.
- Isola, JVV.; et al. Reproductive Ageing: Inflammation, Immune Cells, and Cellular Senescence in the Aging Ovary. Reproduction. 2024 Jun 21;168(2):e230499.
- W, Chuqing.; et al. Hallmarks of Ovarian Aging. Trends Endocrinol Metab. 2025 May;36(5):418–439.
- Cavalcante, MB.; et al. Ovarian Aging in Humans: Potential Strategies for Extending Reproductive Lifespan. Geroscience. 2023 Aug;45(4):2121–2133.
- Xing, X.; et al. Fisetin Delays Postovulatory Oocyte Aging by Regulating Oxidative Stress and Mitochondrial Function through Sirt1 Pathway. Molecules. 2023 Jul 20;28(14):5533.
- Liu, S.; et al. Pyrroloquinoline quinone promotes human mesenchymal stem cell-derived mitochondria to improve premature ovarian insufficiency in mice through the SIRT1/ATM/p53 pathway. Stem Cell Res Ther. 2024 Apr 5;15(1):97.
- Kim, SP, et al. Black Ginger Extract Suppresses Fat Accumulation by Regulating Lipid Metabolism in Mice Fed a High-Fat Diet. J Med Food. 2024 Oct;27(10):922–930.
- Miraglia, N.; et al. Folate Supplementation in Fertility and Pregnancy: The Advantages of (6S)5-Methyltetrahydrofolate. Altern Ther Health Med. 2022 May;28(4):12-17.
- Ekiz, E.; et al. Exploring the Potential Medicinal Benefits of Ganoderma lucidum: From Metabolic Disorders to Coronavirus Infections. Foods. 2023;12(7):1512.