In recent years, Akkermansia muciniphila has moved from a promising microbiome signal to one of the most closely watched next-generation probiotics – particularly in the context of metabolic health.
Yet for product formulators, interest has often outpaced feasibility. Questions around strain viability, enumeration, regulatory positioning, and – most critically – human clinical relevance have limited its practical application in finished products.
That equation may now be changing. New human clinical data on a proprietary live Akkermansia muciniphila (NūGensia®) strain, produced using an advanced encapsulation and fermentation approach, suggests measurable improvements across multiple markers of glycemic and cardiometabolic health. Importantly, these outcomes are tied to a formulation-ready, oxygen-protected strain that remains stable under commercial storage conditions – addressing a long-standing barrier to market adoption.
Recent clinical findings, combined with advances in strain stabilization and enumeration, are expanding what is now possible for formulators developing credible glucose-management and metabolic-health solutions using live Akkermansia. More than 1.5bn men and women worldwide suffer from a type of metabolic impairment.¹ In recent years, it has become clear that the gut microbiota can play a major role in metabolic health.²
Growing market for glucose-management solutions
A 2025 report indicates that the global glucose management supplement market is expected to expand from an estimated $9.9bn in 2025 to $15.4bn in 2032, with an estimated CAGR of 6.4%.³
“With the growing market for supplements designed to focus on glucose management, it’s no surprise that the Akkermansia muciniphila market is also growing at a healthy pace,” says Steve Geiger, vice president, sales and operations for Vidya’s US division.
The global Akkermansia muciniphila market was estimated to be valued at over $159 m in 2025, and is projected to exceed $271m by 2032, through a CAGR of 7.86%.⁴ Other reports suggest the probiotic is poised for even greater growth, with an estimated CAGR of 8.54% by 2032.⁵
Clinically-validated results
As part of its ongoing research efforts, Vidya (Bunnell, Florida) developed a proprietary Akkermansia muciniphila strain, NūGensia (VHAKM) and has evaluated its effects across multiple human clinical studies. The VHAKM strain was recently the subject of a double-blind, randomized, placebo-controlled trial that demonstrated Akkermansia muciniphila’s ability to serve metabolic health, and more specifically, blood sugar management.
In the trial, subjects were recruited across leading hospitals in both Bangalore and Chennai, India. Subjects were administered one capsule daily containing 10bn Colony Forming Units (CFU) of live Akkermansia muciniphila VHAKM for 60 days to understand the effects of the novel probiotic on markers of metabolic health.
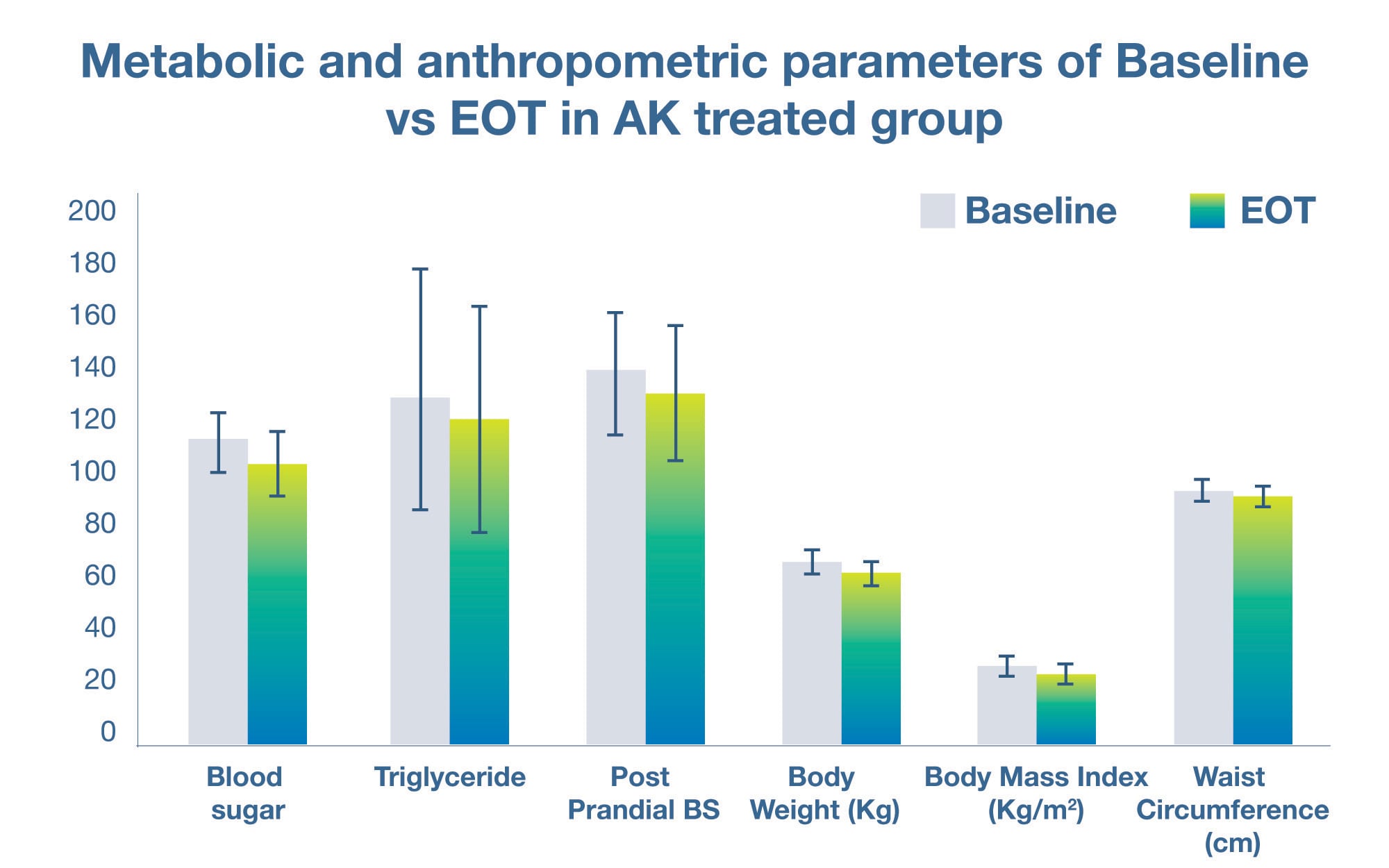
On measures of glycemic health, it was found that the probiotic group experienced a significant improvement in fasting blood sugar, postprandial blood sugar, and HbA1c in comparison to the placebo group (p < 0.05). There was also a concomitant positive change in markers of cardiovascular health, with a significant decrease in triglycerides and diastolic blood pressure in the probiotic group as compared to the placebo group (p < 0.05).
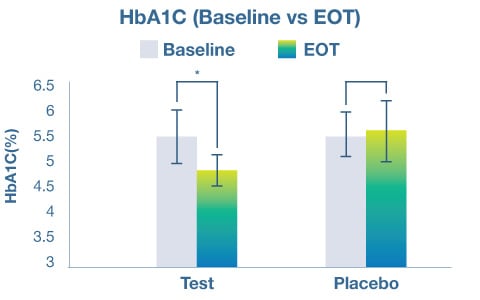
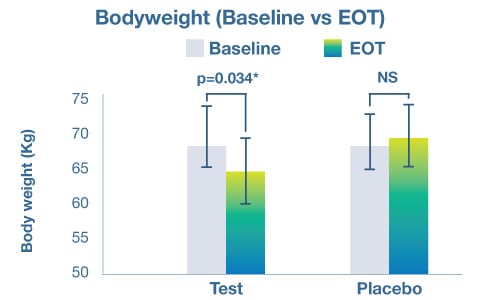
Lastly, the study revealed that the probiotic group had superior improvements in anthropometric variables, including body weight, body-mass-index (BMI) and waist circumference. The probiotic material was found to be well tolerated by subjects with no adverse events being attributed to its consumption.
The study confirmed the ability of Akkermansia muciniphila VHAKM to support weight management as well as metabolic health, as evidenced by the numerous improvements in markers of both glycemic and cardiovascular health.
“These findings establish Akkermansia muciniphila as a clinically validated, next-generation probiotic for metabolic health, offering a natural and effective strategy to support glucose balance, weight management, and overall metabolic wellbeing,” says Subhendu Nayak, director of R&D (probiotics) at Vidya.
“We are excited to collaborate with customers on how to formulate this next generation probiotic into their formulas for metabolic health, as well as other health benefit categories we are evaluating for this material.”
Overcoming barriers to commercial applications
A key factor supporting the observed clinical benefits is the strain’s stability and verified live cell count at the administered dose – an aspect historically limited by Akkermansia muciniphila’s sensitivity to oxygen and a major barrier to its commercial application.
To that end, Vidya developed a specialized manufacturing method to encapsulate it’s proprietary strain, VHAKM, in a starch-based shell to protect the material from oxygen during production and storage.
This encapsulation technology allows for stability to be maintained for up to 24 months under proper storage and packaging conditions. The strain is fermented using a plant- based gum rather than animal derived mucin, allowing for vegan and vegetarian label claims. The fermentation and encapsulation process are the subject of a pending patent.
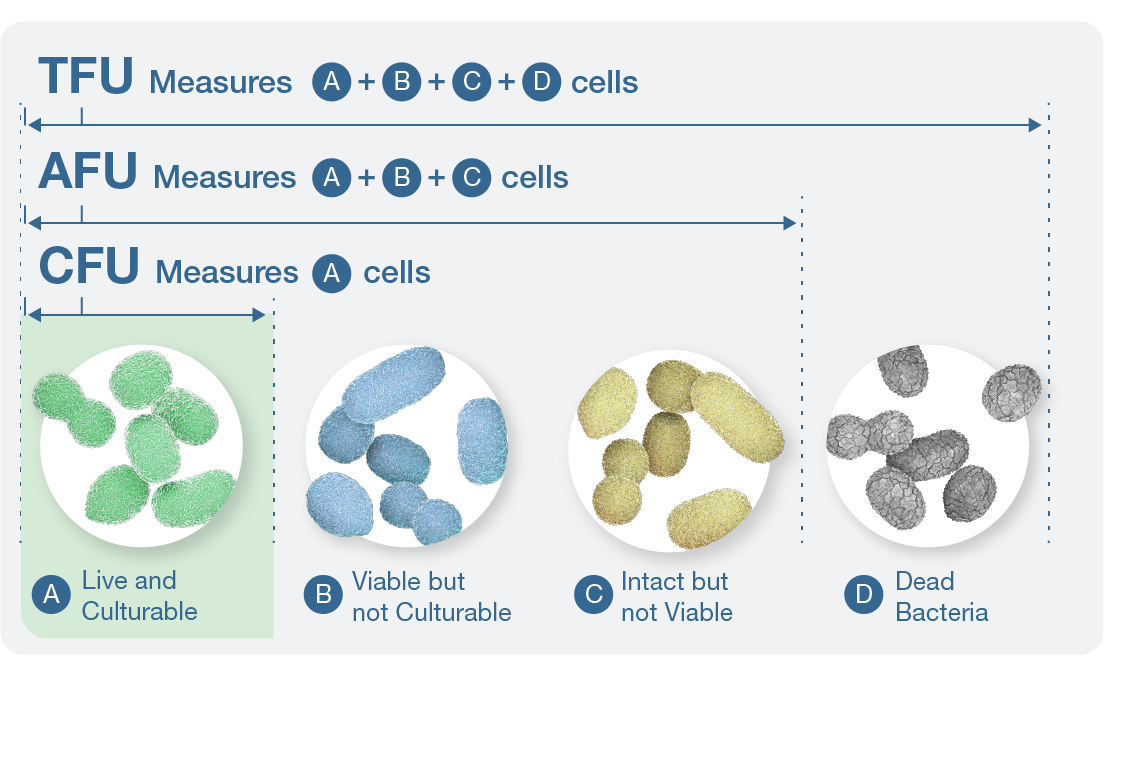
The stability of the Akkermansia muciniphila can only be accurately assessed when using the proper enumeration method to quantify the presence of live, biologically active probiotic bacteria. The gold standard for reporting this is using CFU, which only report on live, culturable cells. Other methods of enumeration include:
- Active fluorescent units (AFU) A measure of cells assumed to be viable
- Non-active fluorescent units (n-AFU) A measure of cells that are dead or damaged
- Total fluorescent units (TFU) A measure of all cells
However, CFU is the best measure when enumerating live Akkermansia muciniphila to ensure consumers are getting a serving equivalent to that used in clinical research.
The recent clinical findings on Akkermansia muciniphila VHAKM signal a shift from emerging microbiome research to formulation-ready application in the metabolic health sector, supported by human data.
Advances in strain stabilization, validated enumeration, and oxygen-controlled manufacturing address key barriers that have historically limited commercial use. With both clinical validation and demonstrated stability now in place, these developments open new opportunities for the introduction of Akkermansia-based products across glucose management and broader metabolic-health applications.
References
- Noubiap, J.J.; et al. Worldwide trends in metabolic syndrome from 2000 to 2023: a systematic review and modelling analysis. Nat Commun. 2026; 17, 573.
- Peng-Xu, W.; et al. Gut microbiota and metabolic syndrome. Chinese Medical Journal. 2020; 133(7):p 808-816.
- Persistence Market Research. Glucose Management Supplements Market Share and Trends Analysis.
- 360iResearch. Akkermansia Muciniphila Market by Product Type.
- Research and Markets. Akkermansia Muciniphila Market - Global Forecast 2025-2032.





