An infant’s health from birth establishes the foundation for wellbeing throughout their life.
As global populations age, and notably so in Europe, which is predicted to see close to half a million centenarians in the EU-27 countries by 2050, promoting infant health can be considered an essential practise for investing in healthier adult populations and reducing future healthcare burdens.¹
Gut health plays a key role in an infant’s overall development. A balanced and healthy gut microbiome supports nutrient absorption, helping infants thrive. It is known that breast-fed infants and formula-fed infants have different gut microbiota and breast-fed infants have a higher abundance of Bifidobacteria. Establishing healthy gut microbiota – such as with Bifidobacteria – from birth is associated with reducing the risk of food allergy sensitisation which starts in the early months of an infant’s life.
Type of birth delivery can also affect the composition of Bifidobacteria in the infant gut and can have health effects into adulthood. During vaginal births, Bifidobacteria are vertically transmitted from the mother’s birth canal and colonise the intestine of the infant during the very early stages of life.
Babies delivered by C-section can miss out on exposure to this beneficial bacteria. Here, the abundance of Bifidobacterium is lower than vaginal births and tends to have delayed colonisation. Globally, the rate of C-sections has tripled since 1990 to 21% in 2021, with the procedure viewed as essential in situations where prolonged or obstructed labour, fetal distress or because the baby is presenting in an abnormal position can pose serious or life-threatening risk to both mother and baby.²
Feeding mode is another major factor that can impact the colonisation level and species composition of Bifidobacteria in an infant’s gut. Early exposure to a balanced diet during infancy creates a strong foundation to help reduce the risk of gut-related health issues or chronic diseases in adulthood and older age.
Breast milk provides essential nutrients that support optimal growth, immune function and cognitive development. The World Health Organization (WHO) recommends exclusive breastfeeding for the first six months of an infant’s life, followed by continued breastfeeding with complementary foods for up to two years and beyond.³
However, many women face challenges with breastfeeding due to the demands of balancing work and family responsibilities and, globally, many infants and children do not receive optimal feeding. In Europe, breastfeeding rates are considered low, especially in the UK which has one of the lowest breastfeeding rates globally, with only 0.5% of babies breastfed up to one year.⁴
To bridge the gap between the realities of modern life and prioritising their infant’s nutrition, parents may seek alternative solutions to breastfeeding, such as formula supplementation.
Promoting gut health in infants for lifetime health
Human-Residential Bifidobacteria (HRB) are the predominant beneficial bacteria that colonise the infant gut and are involved in early life immune development. A well-established HRB population is associated with stronger immunity and long-term gut health benefits, including lower risk of allergies, obesity and metabolic disorders.
A 2024 study found that when Bifidobacterium was the predominant HRB in infants aged one month old, there was a reduced rate of egg white allergen sensitisation and food allergies in the future.⁵
Another key role of Bifidobacteria is to build the foundation for health and support early life immune development. Studies have shown that Actinobacteria (predominantly B. infantis) abundance was positively associated with T cell responses to different vaccinations, such as BCG (Bacillus Calmette-Guérin), OPV (Oral Poliovirus Vaccine) and TT (Tetanus Toxoid) which are typically administered in infancy and childhood.⁶
Breastmilk supports the establishment and persistence of the specific group of HRB. When comparing the gut microbial composition in infants with different feeding modes, breastfed infants’ guts are dominated with Bifidobacteria. In contrast, formula-fed infants had a much lower proportion of Bifidobacteria.
Therefore, supplementing specific Bifidobacteria strains can be beneficial to help prevent dysbiosis and other related diseases later in life.
The link between HRB and HMO
Human Milk Oligosaccharides (HMO) are a unique type of complex carbohydrate found in human breast milk. Despite being the third most abundant solid component in breast milk (after lactose and fats), infants are unable to digest and absorb HMOs.
One of the important characteristics of HRB is their capability to utilise HMOs. When HMOs reach the gut, they are broken down by and become food for Bifidobacteria. This promotes the growth of healthy gut bacteria.
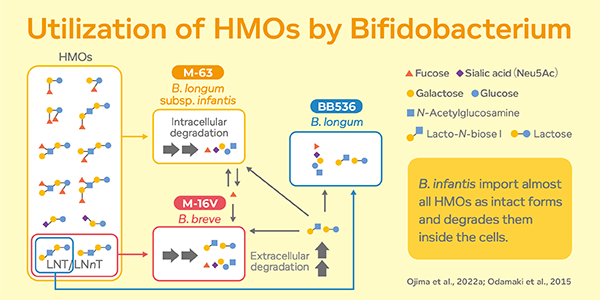
However not all Bifidobacterium species are able to colonise and establish in the infant’s gut. B. bifidum, B. infantis and B. breve are HRBs which have a high ability to utilise HMOs. B. infantis imports almost all HMOs as intact forms and degrade them inside the cells. During this process, part of the monosaccharides is expelled from the cell, which is then preferentially utilised by B. breve to outcompete often the other species in infant intestine.
Of HRBs, Bifidobacterium longum subsp. infantis M-63 has shown superiority in utilising different types of HMOs.
Scientifically-validated probiotic strain for infant health
Morinaga Milk has developed a portfolio of HRB probiotic strains that are highly compatible with the human gut. The clinical efficiency of its M-63 strain is supported by 36 scientific studies (as of March 2025).
In 2023, a RCT study investigated the effects of M-63 on infants. During the study, 110 infants were given a daily dose of one billion colony forming units (CFU) for three months.⁷
Results showed that M-63 significantly increased the relative abundance of Bifidobacteria from the first week compared to placebo and promoted the establishment of Bifidobacteria at one week and one month regardless of C-section or vaginal births. Feeding type did not alter the effectiveness of probiotics in promoting early colonisation with Bifidobacteria. Further, increased SCFA levels, decreased stool pH, and increased immunoglobulin A (IgA) concentration has been shown in faecal samples of the M-63 group.
Given that even some exclusively breastfed infants had lower levels of Bifidobacteria in their gut, supplementation with probiotic M-63 may be beneficial for breastfed and mixed-fed infants and a consideration for formula-fed infants.

The study also revealed the beneficial effects of M-63 on Bifidobacterial colonisation and the intestinal environment. Such colonisation by commensal Bifidobacteria during early life is indispensable for the normal development and growth of the gastrointestinal tract, particularly for epithelial barrier function and mucosal immunity.⁸
M-63 supplementation was also shown to promote the frequency of bowel movements and watery bowel movements decreased, suggesting early supplementation with M-63 can have beneficial effects on intestinal development. This is particularly important during early infancy.
Preventing allergy development
M-16V and BB536 are two other clinically effective HRB probiotic strains produced by Morinaga Milk. A 2014 study investigated the effects of Bifidobacterial supplementation as a useful method for preventing allergies in infants.⁹

In an open trial, Bifidobacterium breve M-16V and Bifidobacterium longum BB536 were supplemented prenatally to 130 mothers one month prior to delivery and postnatally to the infants for 6 months – with 36 mother-infant pairs serving as the control group. Development of allergic symptoms in infants was assessed at months 4, 10 and 18 and faecal samples were collected from the mothers and infants.
Results showed that developing eczema/atopic dermatitis during the first 18 months of life was significantly reduced in infants in the probiotic group. Pyrosequencing analyses highlighted an altered composition of the faecal microbiota at four months for infants who developed eczema/AD at four and 10 months. The proportion of Proteobacteria in mothers at the time of delivery was also significantly lower compared to the control group.
The study concluded that pre and postnatal supplementation of Bifidobacteria is effective in preventing allergic diseases.

Unlocking early-life gut health
Morinaga Milk realised the potential of probiotics in gut microbiota development thanks to its work into the broader role of HMOs. By promoting the importance of HMOs and Bifidobacteria into infant formulas, the company aims to replicate the natural gut-nourishing effects of breast milk and help promote Bifidobacterium colonisation.
Exploring the safety, functional health benefits and mechanisms of action can provide a greater understanding of HRBs and their role in human health. Prioritising evidence-based solutions helps bring improved health to infants, supporting wellness throughout their lifespan.
References
- Eurostat. Ageing Europe - statistics on population developments.
- World Health Organization, June 2021. Caesarean section rates continue to rise, amid growing inequalities in access.
- World Health Organization. Breastfeeding.
- NHS Digital. Infant Feeding Survey - UK, 2010.
- Coyle, D. H.; et al. (2024). An Evaluation of the Nutritional and Promotional Profile of Commercial Foods for Infants and Toddlers in the United States. Nutrients, 16(16), 2782.
- Shibata, R.; et al. (2025). Neonatal gut microbiota and risk of developing food sensitization and allergy. Journal of Allergy and Clinical Immunology, 155(3), 932-946.
- Huda, M. N.; et al. (2024). Stool microbiota and vaccine responses of infants. Pediatrics, 134(2): e362-72.
- Hiraku, A.; et al. (2023). Early Probiotic Supplementation of Healthy Term Infants with Bifidobacterium longum subsp. infantis M-63 Is Safe and Leads to the Development of Bifidobacterium-Predominant Gut Microbiota: A Double-Blind, Placebo-Controlled Trial. Nutrients, 15(6), 1402.
- Enomoto T.; et al. (2014). Effects of bifidobacterial supplementation to pregnant women and infants in the prevention of allergy development in infants and on fecal microbiota. Allergol Int.








