Human Milk Oligosaccharides (HMOs) are transforming the landscape of early life nutrition, moving from a scientific curiosity to a cornerstone of advanced infant formula.
These complex carbohydrates, the third most abundant solid component in human milk, support various elements of infant health including immunity, gut microbiome development and cognitive function. As consumer demand for science-backed, high-quality ingredients grows, meeting this demand requires more than just ingredients – it requires a partner with proven expertise, a global regulatory footprint and a portfolio designed for market leadership.
Novonesis is at the forefront, as the first company to commercialize HMOs in 2015 and now leading regulatory advancements and expanding access to these valuable ingredients across the Asia-Pacific region.
This article explores the significant steps Novonesis is taking in the HMO market, from landmark approvals in key APAC countries to a forward-thinking global regulatory strategy. It will examine how these developments empower brands to deliver next-generation infant nutrition that is closer to nature.
A new era of HMO access in Singapore
Singapore has long been a benchmark for innovation and stringent food safety standards in Asia. The recent approval by the Singapore Food Agency (SFA) for 3′-Sialyllactose (3′-SL) and 6′-Sialyllactose (6′-SL) completes the regulatory clearance for Novonesis’ full MyOli® 5HMOMIX.
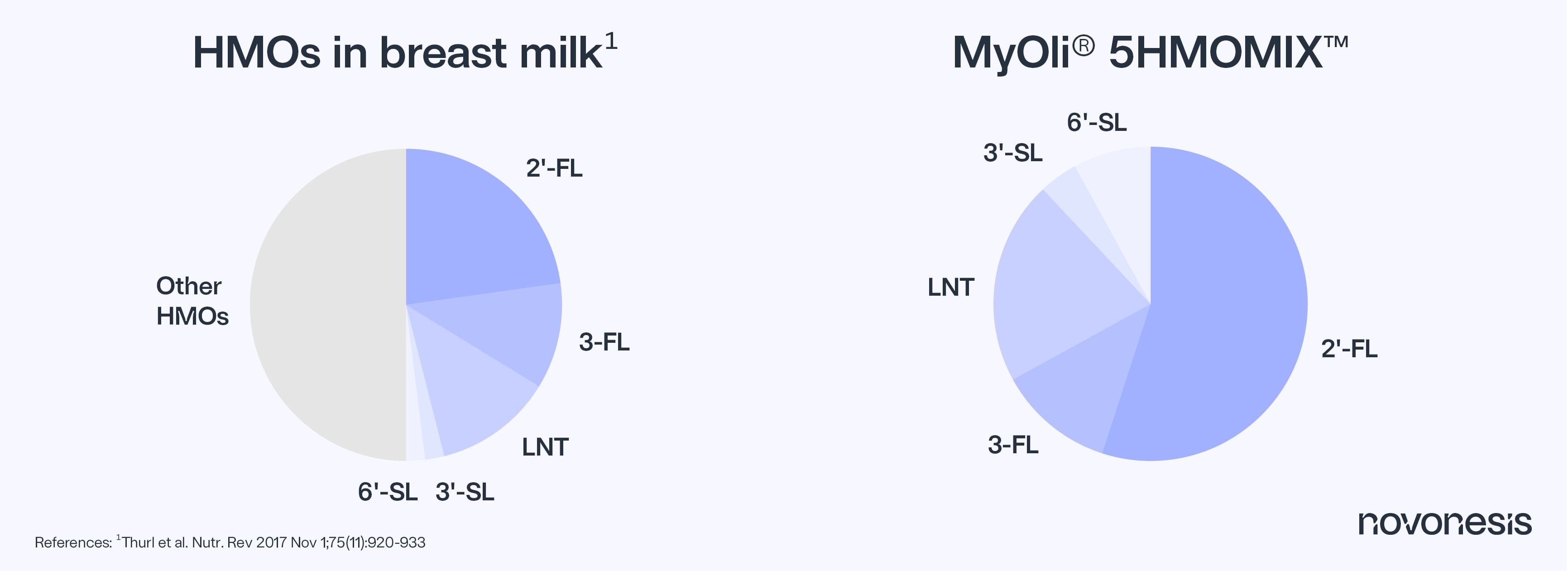
This important authorization positions Novonesis as the only supplier providing access to all five distinct HMOs, both individually and as a complete blend: 2′-Fucosyllactose (2′-FL), 3-Fucosyllactose (3-FL), Lacto-N-tetraose (LNT), 3′-SL and 6′-SL.
The MyOli® 5HMOMIX resembles the natural composition of human breast milk. The blend consists of the five key HMOs that together make up 30-50% of the total found in breast milk and covers all structural groups of HMOs known.
By achieving this full-spectrum approval, Novonesis provides manufacturers with the tools to get closer to the complexity of HMOs found in human milk. This development enables brands to create advanced products that offer a wider range of benefits for infant health and development.
Furthermore, the recent approvals extend beyond infant formula, with the full MyOli® 5HMOMIX now authorized for use in the Growing Up Milk (GUM) category. This expansion opens new avenues for product innovation, allowing for the inclusion of specific HMO benefits in a broader range of products for toddlers and young children.
Building on a foundation of global regulatory success
The recent achievements in APAC are built upon a solid foundation of global regulatory milestones. The MyOli® 5HMOMIX has been authorized for use in infant nutrition and beyond in the US since 2021 and the EU since 2023.
These established approvals in highly regulated markets underscore the safety, quality and efficacy of Novonesis’ HMO portfolio, providing a trusted framework for global expansion.
This momentum has continued into 2025 with significant progress in other key markets:
- Canada In March 2025, Health Canada authorized the MyOli® 5HMOMIX as a mixture for use in infant formulas and toddler formulas.
- India Also in March 2025, the MyOli® 5HMOMIX was approved for use in infant formula. As a major market within the broader APAC region, this approval significantly expands access to advanced nutrition for millions of infants.
These harmonized global approvals create a clear path for innovation. By enabling formulation with a consistent, reliable and clinically proven blend of HMOs, they empower brands to deliver high-quality nutrition to consumers worldwide.
Expanding market access across the APAC region
Beyond these recent landmark approvals, Novonesis has already established significant market access for 2′-FL, one of the most abundant HMOs in human milk. This foundational ingredient is already available in numerous APAC markets, including:
- China
- Australia
- New Zealand
- Malaysia
- Taiwan
This established presence provides a solid platform for introducing more complex HMO blends. The path is already paved for manufacturers in these countries to innovate and enhance their product lines with scientifically supported ingredients.
Further opportunities for market entry are emerging in Vietnam and Hong Kong. In these regions, the established US or EU regulatory status can be leveraged to facilitate the registration of customer products containing MyOli® HMOs. This pathway provides a strategic advantage for brands looking to enter or expand their presence in these dynamic markets, accelerating speed to market for innovative nutritional solutions.
The future of HMOs in APAC: A commitment to progress
The journey of HMO innovation is continuous. Novonesis is actively working to secure approvals for its complete MyOli® 5HMOMIX in several other key APAC countries. While the specifics of regulatory timelines are complex and depend on many factors, the direction is clear: to make the full benefits of HMO science accessible across the entire region. This forward-looking commitment ensures that partners can plan for future growth and stay ahead of consumer expectations.
“We understand that navigating the evolving regulatory landscape can be challenging. Our expertise is not just in producing high-quality HMOs, but also in providing the regulatory support and market insights our partners need to succeed. We are committed to fostering innovation and helping you bring products to market that make a real difference in people’s lives,” says Chandrika Balachandrun, Head of Regulatory Affairs APAC at Human Health Biosolutions.
The progress in the APAC region demonstrates a growing recognition of the profound impact of HMOs on infant and children’s health. As science continues to uncover the intricate benefits of these powerful ingredients, Novonesis remains dedicated to leading the way. In addition to supplying ingredients, Novonesis is partnering with clients to shape the future of early life nutrition and help to grow your market.
“At Novonesis, our mission is to bridge the gap between groundbreaking science and accessible, advanced early life nutrition solutions. Our regulatory advancements in the APAC region underscore our commitment to enabling brands to deliver products that truly are inspired by nature,” Balachandrun continues.
For further information on its HMO portfolio and navigating the regulatory landscape in your specific market, reach out to experts at Novonesis here. Partnering with Novonesis can unlock new opportunities and deliver the next generation of science-backed nutrition to families across Asia-Pacific.
*The World Health Organization (WHO) recommends breastfeeding exclusively for the first six months, followed by continued breastfeeding together with complementary foods. Novonesis fully supports breastfeeding as the best option for the start of life. But for some mothers and infants, breastfeeding is not an option. Here, HMOs can support in bringing an alternative formula option closer to mother’s milk.


