In 2023, only 14% of consumers were familiar with ashwagandha and had incorporated it into their wellness routines.¹
Today, that number has doubled.¹ By 2028, ashwagandha supplement sales are projected to reach an impressive $691m, positioning it to surpass turmeric and emerge as the top-selling herbal supplement.¹
Much of ashwagandha’s success can be attributed to its mood- and stress-management benefits. In fact, ashwagandha supplements marketed for mood and stress benefits account for 77% of the ingredient’s sales.¹
Additionally, the global adaptogen drink market is projected to reach $3.3bn by 2033, driven by similar consumer trends that are influencing the dietary supplement market.² Within the adaptogen drink market, stress management remains a primary concern, with 37.2% of product applications centered around it.² Notably, ashwagandha stands out as a key ingredient in this market, commanding 35% of the adaptogen drink market share.²
However, with this high consumer demand comes significant formulation challenges. Many ashwagandha extracts currently on the market face issues such as poor absorption, lack of scientifically-validated standardization and undesirable taste profiles, all of which can hinder product success.
This article outlines the five most common challenges associated with ashwagandha extracts and present effective strategies for overcoming these obstacles. By addressing these issues – dietary supplement, functional food and functional beverage – brand manufacturers can enhance product quality and meet the growing consumer demand for reliable, effective and enjoyable ashwagandha products.
Challenge #1: Ashwagandha bioavailability
Ashwagandha (Withania somnifera), a prominent herb revered for its numerous health benefits, contains hundreds of compounds. Specifically, withanolides within Withania somnifera are known as the primary active compounds responsible for the plant’s pharmacological effects. Recent research from Arjuna Natural introduces a groundbreaking discovery: withanolides exist in two distinct forms – withanolide aglycones and withanolide glycosides, also known as glycowithanolides.³
Understanding these forms is crucial for formulators aiming to optimize product efficacy. Withanolide aglycone is the free withanolide form. Whereas the glycowithanolide form is where the bioactive molecule (withanolide) is bound to a sugar molecule. Each form can have varying levels of bioavailability and effectiveness in the body.
In a recent randomized, double-blind, crossover pharmacokinetic (PK) study, it was identified that an extract standardized to 35% withanolide glycosides (Shoden) demonstrated:
- Exceptional bioavailability – 226 times more effective than the leading ashwagandha extract.
- Cmax (Maximum concentration) levels six times higher than those of other ashwagandha extracts.
- The ability to stay in the body for over 20 hours (four times more than other ashwagandha ingredients).
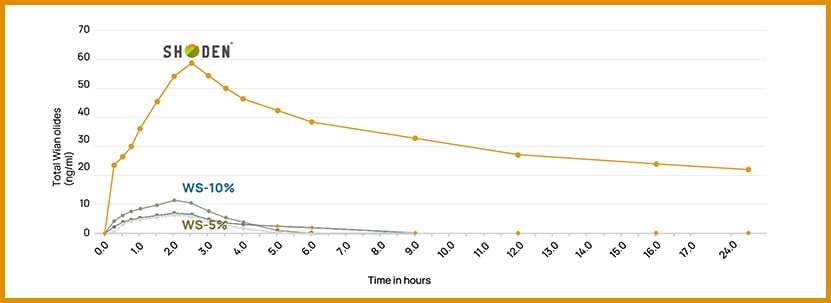
It is important to note that equivalent dosages were administered among the four ashwagandha extracts tested in the study. The primary difference was the attachment of the sugar molecule in Shoden, which led to improved water solubility and absorption, allowing the compound to pass through the digestive tract more efficiently.
Pro tip: When choosing an ashwagandha extract, choose the extract made with the more bioavailable form of withanolides (withanolide glycosides).
Challenge #2: Ashwagandha standardization
When shopping online for ashwagandha supplements, there are a wide range of formulas available. Some with root powder, some with leaf powder, some standardized to a 40:1 root-to-leaf extract, and some standardized to 5% to 10% total withanolides, leaving consumers confused about the ideal extract.
The situation is further complicated by the limitations of the existing testing methods. While a United States Pharmacopeia (USP) standardized method for testing withanolides exists, it can only identify a limited set of compounds (eight to 10) in ashwagandha.
Recognizing these limitations, Arjuna Natural has pioneered and published a high-performance liquid chromatography (HPLC) method that quantifies a large number of Withania somnifera molecules. Seen by many supplement brands and International Organization for Standardization (ISO) accredited labs as the gold standard of ashwagandha testing, Shoden Ashwagandha is standardized to the highest withanolide concentration (minimum of 35% withanolide glycosides) and proven using a validated HPLC testing method.
Testing tip: Leverage an ISO-accredited third-party testing lab to test the ashwagandha extract you’re looking to supply. Third-party certifications and testing have a significant impact on driving consumer purchases of supplements.
Challenge #3: Ashwagandha dosage
When it comes to ashwagandha extracts, the conventional approach of recommending a daily intake of two large capsules (500-1000mg) is falling out of favor, particularly among younger consumers. Gen Z and millennials are increasingly gravitating towards more enjoyable and convenient supplement delivery formats, such as gummies, teas and functional foods and beverages. Particularly, gummies have emerged as the top choice for 60% of Gen Z users, while tea ranks as the second-most-preferred method among millennials for consuming their supplements.¹
Recognizing this shift in consumer preferences, Arjuna Natural has innovated with the launch of Shoden-R. This root-only extract delivers proven benefits for stress relief, relaxation and cognitive health at a low daily dose of just 60mg.5-7
Additionally, a recent double-blind, three-arm, placebo-controlled study revealed no significant clinical difference between the traditional root and leaf extract (Shoden) and the new root-only extract (Shoden-R) offered by Arjuna Natural.7 For brand manufacturers, this means that the 11 international studies showcasing the safety and efficacy of Shoden at a dose as high as 480mg and a clinically proven low daily dose of 60mg can directly apply to Shoden-R.⁵⁻¹⁶

Market insight: Consumers are seeking alternative ways (excluding alcohol) to unwind and relax at night. Try the ashwagandha (Shoden-R), recently awarded the SupplySide Global Award for the ‘Food & Beverage Relaxation’ category.
Challenge #4: Ashwagandha organoleptic properties
Ashwagandha tends to have a strong, earthy flavor that can be a turn-off for many consumers. Additionally, the mix of root and leaf can create a bitter taste. With the growing trend towards gummies, chocolates and beverages for supplement consumption, the current ashwagandha organoleptic properties are less than ideal for many brand manufacturers.
The root-only format of Shoden (Shoden-R) eliminates the bitter tones typically associated with root and leaf extracts. Additionally, Shoden-R is 100% water-soluble, odorless, nearly transparent and virtually tasteless, making it ideal for functional beverages, gummies and chews.
Formulation tip: Younger consumers are open to and prefer functional food and beverages that offer unique taste experiences. One unique taste experience to test out in the R&D lab is mango iced tea stick packs with Shoden-R in it.
Challenge #5: Ashwagandha safety and efficacy data
Many of the safety and efficacy challenges associated with ashwagandha arise from the use of unsubstantiated and unstandardized extracts, which can compromise product quality and consumer trust. This is why it is essential to use only ashwagandha extracts with proven safety and efficacy data.
Take, for example, recent studies on a 35% withanolide glycoside extract (Shoden). A long-term human safety study demonstrated that this specific standardized extract, when consumed at four to eight times the daily recommended dose, showed no adverse effects and was well-tolerated among study participants.¹⁷
Shoden has also been proven effective in 11 published international trials, showcasing its ability to help the body adapt to stress, improve sleep quality and enhance overall vitality.5-16 More recently, a study published in 2024 found that consuming just 60mg/day of this specific branded ingredient (Shoden) reduced cortisol levels by up to 66% within 60 days, significantly outperforming other ashwagandha extracts, which show a reduction of only 22% to 33%.5 In the same placebo-controlled, double-blinded study, Shoden also reduced anxiety by up to 59%, as measured by the Hamilton Anxiety Rating Scale (HAMA).5
Incorporating Shoden into formulations not only positions brands as one with efficacious ingredients but also addresses consumer demand for scientifically backed dietary supplements.
Market insight: Effectiveness is by far the number one most important factor for consumers when choosing an herbal supplement (over 70%).¹
References
- Nutrition Business Journal. Herbs and Botanicals Report.
- Global Adaptogen Drink Market By Ingredients. Market.us.
- Whitepaper - Data on file.
- Rathi, P.; et al. Randomized, Double-Blind, Crossover Study Comparing the Bioavailability of 4 Ashwagandha (Withania somnifera (L.) Dunal) Extracts in Healthy Adults Under Fasting Condition. Current therapeutic research. 2025; 103, 100805.
- Mishra, D. N.; et al. Shoden promotes Relief from stress and anxiety: A randomized, double-blind, placebo-controlled study on healthy subjects with high stress levels. Heliyon. 2024; 10(17), e36885.
- Dimpfel, W.; et al. Effects of an Adaptogenic Extract on Electrical Activity of the Brain in Elderly Subjects with Mild Cognitive Impairment: A Randomized, Double-Blind, Placebo-Controlled, Two-Armed Cross-Over Study. Pharmaceuticals. 2020; 13(3), 45.
- Shoden-R Latest Study. Submitted for publication.
- Kim, S. K.; et al. Pharmacokinetics and bioequivalence of Withania somnifera (Ashwagandha) extracts - A double blind, crossover study in healthy adults. Heliyon. 2023; 9(12), e22843.
- Murthy, S. V.; et al. Hydroalcoholic Extract of Ashwagandha Improves Sleep by Modulating GABA/Histamine Receptors and EEG Slow-Wave Pattern in In Vitro - In Vivo Experimental Models. Preventive nutrition and food science. 2022; 27(1), 108–120.
- Tharakan, A.; et al. Immunomodulatory Effect of Withania somnifera (Ashwagandha) Extract-A Randomized, Double-Blind, Placebo Controlled Trial with an Open Label Extension on Healthy Participants. Journal of clinical medicine. 2021; 10(16), 3644.
- Haque, I. M.; et al. Role of Standardized Plant Extracts in Controlling Alcohol Withdrawal Syndrome-An Experimental Study. Brain sciences. 2021; 11(7), 919.
- Deshpande, A.; et al. A randomized, double blind, placebo controlled study to evaluate the effects of ashwagandha (Withania somnifera) extract on sleep quality in healthy adults. Sleep medicine. 2020; 72, 28–36.
- Mishra, A.; et al. Efficacy of Ashwagandha and Brahmi extract on alcohol withdrawal syndrome in laboratory rats. Int. J. Pharmacol. 2020; 16: 343-350.
- Lopresti, A. L.; An investigation into the stress-relieving and pharmacological actions of an ashwagandha (Withania somnifera) extract: A randomized, double-blind, placebo-controlled study. Medicine. 2019; 98(37), e17186.
- Lopresti, A. L.; et al. A Randomized, Double-Blind, Placebo-Controlled, Crossover Study Examining the Hormonal and Vitality Effects of Ashwagandha ( Withania somnifera) in Aging, Overweight Males. American journal of men’s health. 2019; 13(2), 1557988319835985.
- Benny, A.; et al. Acute and sub chronic toxicity studies of purified withania somnifera extract in rats. International Journal of Pharmacy and Pharmaceutical Sciences. 2018; 10. 41.
- Safety study. Submitted for publication.







