Women’s health research continues to be a category within the dietary supplement industry that is vastly underrepresented in comparison to research on men.
By one report, the global men’s health supplement market size was $US 75.09 billion in 2024, while the women’s health and beauty supplement market size was $US 57.42 billion in the same year.¹⁻² Despite being relatively comparable market sizes, an audit of research on popular sports nutrition ingredients such as creatine and beta-alanine found that there were approximately eight times fewer studies exclusively on females compared to those exclusively on males.³
Research conducted by private industry on dietary supplements in recent years aims to make progress towards balancing those scales. Gencor Pacific (Hong Kong) developed a proprietary fenugreek (Trigonella foenum-graecum) seed extract as well as an enhanced bioavailability form of palmitoylethanolamide for use in dietary supplements and functional foods with ongoing support for research dedicated to women’s health.⁴
The use of fenugreek beyond ayurveda for female sports nutrition
Fenugreek has a long history of traditional use and is regarded as one of the earliest medicinal plants used in ayurveda and Traditional Chinese Medicine (TCM).⁵ Through an extraction process, the seeds can be purified to achieve a higher concentration of a key bioactive component known as furostanol saponins. It is believed that these provide many of the benefits of fenugreek in relation to hormonal health.
This novel fenugreek extract by Gencor has a strong foundation of research in women’s health, with published data demonstrating its ability to support healthy levels of free testosterone and estradiol in menstruating women.⁶
These changes likely explained the benefits women saw in this clinical trial, with statistically significant improvements in various measures of sexual health, such as sexual arousal and sexual frequency in comparison to a placebo.
Following up on that research, a double-blind, randomised, placebo-controlled trial found that just 600 mg per day of the extract combined with a resistance training program for eight weeks had promising benefits.⁷ The study revealed that the 600-mg group experienced significant increases in leg press, total lean body mass and lean leg mass compared to the placebo (p < 0.05).
Simultaneously, the same group also observed significant reductions in total fat mass and trunk fat mass compared to the placebo (p < 0.05).⁷ The improvements in these measures support the conclusion that this extract is effective in increasing lower body strength as well as supporting a healthy body composition.
The material is ideal for capsules, tablets and even functional foods, such as inclusion in chocolate products, partly due to the availability of a taste-masked grade of the material that overcomes the organoleptic challenges linked to conventional fenugreek extracts.
The case for the use of palmitoylethanolamide in women’s health
Palmitoylethanolamide (PEA) is a fatty acid amide that is endogenous to the human body.⁸ It is released by cells in response to harmful stimuli.⁴ It can also be naturally found in various food sources such as eggs, soy, peanuts and corn.⁹
Through a chemical synthesis process involving both palmitic acid and monoethanolamine, a bioequivalent PEA can be produced for use in dietary supplements and functional foods. Most of the benefits of PEA stem from its ability to support a balanced inflammatory response.
However, a significant challenge of supplementing with PEA has been its bioavailability. PEA is considered a lipophilic substance and, as a result, has poor solubility in aqueous (water-based) solutions, which likely explains how oral ingestion results in limited systemic exposure for a short duration.¹⁰ However, a unique excipient platform by Pharmako Biotechnologies (Australia) – LipiSperse® can be applied to PEA to make a form that is almost twice as bioavailable as unformulated PEA.⁴
The excipients coat the lipophilic PEA to create repulsive forces between the particles, reducing aggregation while also lowering surface tension, which in turn allows the liquid to adhere. This transforms the PEA into a cold-water dispersible material with a low excipient load of just 10%.
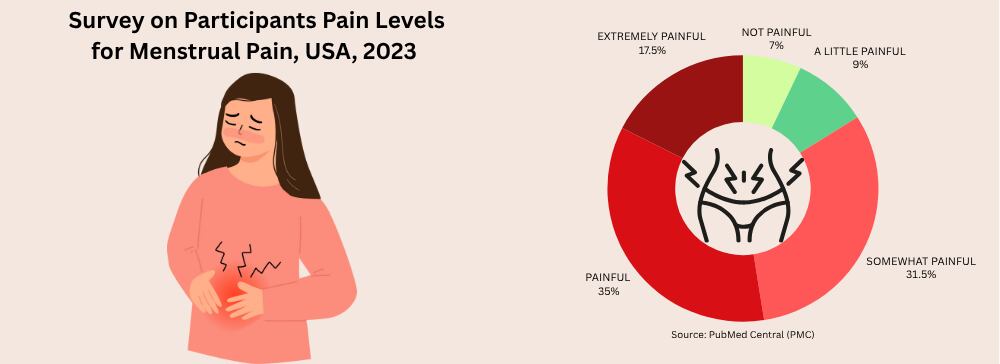
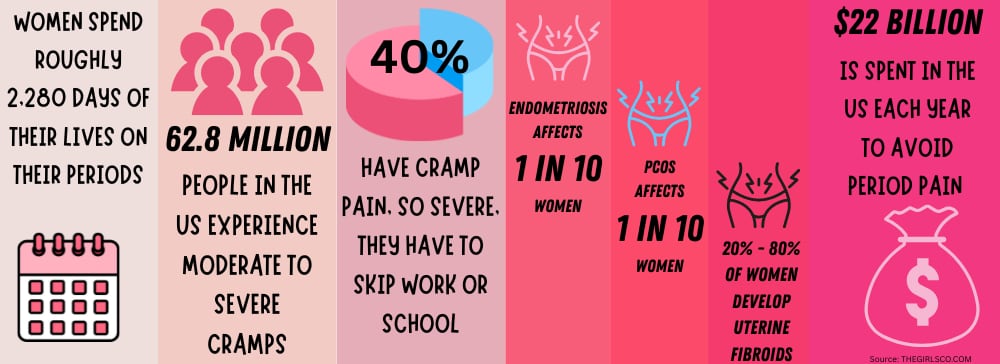
A 2012 study found that a staggering 84.1% of young women suffered from menstrual discomfort, with 31.9% of young women having an association between their menstrual discomfort and absenteeism (e.g., missing study or social activities).¹¹
A 2025 study on the improved absorption of PEA demonstrated promising effects on menstrual discomfort.¹² In this double-blind, randomised, placebo-controlled trial, women experiencing mild to moderate discomfort associated with menstruation were assigned to take either 350 mg of Levagen®+ or a matching placebo at the onset of menstrual discomfort, with an additional dose permitted two hours after onset if discomfort persisted.
The study allowed for the recording of up to four menstrual discomfort events over a four-month period. The results indicated that the PEA group experienced a significant reduction in menstrual discomfort compared to the placebo as early as one hour after ingestion, and this benefit over the placebo persisted for up to 2.5 hours after ingestion.¹²
The study highlights yet another application of Levagen®+ in addressing bodily discomfort. Earlier studies have demonstrated benefits in reducing occasional joint discomfort, nerve discomfort and two studies have examined occasional head discomfort.¹³⁻¹⁶
Given that the improved material is cold-water dispersible, the formulation applications of the material lend themselves not only to conventional capsules, tablets and soft gel formats but also to gummies, ready-to-mix (RTM) powders, stick packs and functional foods.
These clinically studied ingredients for women’s health can help meet the growing demand for health benefits in these categories for women in dietary supplements.
These findings provide healthcare professionals, researchers and formulators with an ever-increasing toolkit of nutritional strategies designed to enhance the everyday lives of women, whether they are managing cycle-related discomfort or pursuing performance goals.
Nutritional interventions for women’s health are increasingly recognised for their ability to provide targeted, non-pharmaceutical support. An analysis of the women’s hormonal supplements market predicted that from 2024 to 2032 the sector may grow at a compounded annual growth rate CAGR of 7.6%, while the female sports nutrition supplement market may expand at a CAGR of 8.8% from 2026 to 2033.¹⁷⁻¹⁸
The clinical data on a bioavailable form of PEA and standardised fenugreek extract reflect a shift toward personalised, evidence-informed approaches to women’s health. Bioavailable PEA and standardised fenugreek extract continue to expand, presenting new opportunities to address longstanding issues such as menstrual pain and hormonal imbalance.
Further studies with larger sample sizes and longer durations are necessary to confirm these findings and explore additional applications.
References
- Grand View Research. Men’s Health Supplements Market Trends.
- Grand View Research. Market Size & Trends.
- Smith, E. S.; et al. (2022). Auditing the Representation of Female Versus Male Athletes in Sports Science and Sports Medicine Research: Evidence-Based Performance Supplements.Nutrients, 14(5), 953.
- Briskey, D.; et al. (2020). Increased Absorption of Palmitoylethanolamide Using a Novel Dispersion Technology System (LipiSperse). Nutraceuticals Food Sci, Vol.5 No.2:3.
- Dr. Chandran.; et al. (2020). The Power of Ayurvedic Spices. Health Secrets from Ancient India.
- Rao, A.; et al. (2015). Influence of a Specialized Trigonella foenum-graecum Seed Extract (Libifem), on Testosterone, Estradiol and Sexual Function in Healthy Menstruating Women, a Randomised Placebo Controlled Study. Phytotherapy Research, Vol 29, Issue 8.
- Rao, A.; et al. (2023). Libifem® (Trigonella foenum-graecum) in conjunction with exercise on muscle strength, power, endurance, and body composition in females aged between 25 and 45 years. Front. Sports Act. Sec. Sport and Exercise Nutrition, Vol 5.
- Bountra, C.; et al. (2019). Current Understanding, Emerging Therapies, and Novel Approaches to Drug Discovery. Pain.
- Peritore, A. F.; et al. (2019). Therapeutic Efficacy of Palmitoylethanolamide and Its New Formulations in Synergy with Different Antioxidant Molecules Present in Diets. Nutrients, 11(9), 2175.
- Vacondio, F.; et al. (2015). Amino Acid Derivatives as Palmitoylethanolamide Prodrugs: Synthesis, In Vitro Metabolism and In Vivo Plasma Profile in Rats. Plos One.
- Grandi, G.; et al. (2012). Prevalence of menstrual pain in young women: what is dysmenorrhea? Dovepress Taylor & Francis Group.
- Rao, A.; et al. (2025). Palmitoylethanolamide (Levagen+) for acute menstrual pain: a randomized, crossover, double-blind, placebo-controlled trial. Women & Health, 65(3), 237–245.
- Briskey, D.; et al. (2020). The Effect of a Dispersible Palmitoylethanolamide (Levagen+) Compared to a Placebo for Reducing Joint Pain in an Adult Population – A Randomised, Double-Blind Study. International Journal of Nutrition and Food Sciences. Vol 10, Issue 1.
- Pickering, E.; et al. (2022). A randomized controlled trial assessing the safety and efficacy of palmitoylethanolamide for treating diabetic-related peripheral neuropathic pain. Inflammopharmacol 30, 2063–2077.
- Briskey, D.; et al. (2022). Efficacy of Palmitoylethanolamide (Levagen+TM) Compared to Ibuprofen for Reducing Headache Pain Severity and Duration in Healthy Adults: A Double-Blind, Parallel, Randomized Clinical Trial.Food and Nutrition Sciences. Vol.13 No.7.
- Briskey, D.; et al. (2024). Effectiveness of Palmitoylethanolamide (Levagen+) Compared to a Placebo for Reducing Pain, Duration, and Medication Use during Migraines in Otherwise Healthy Participants—A Double-Blind Randomised Controlled Study. Pharmaceuticals, 17(2), 145.
- SNS Insider. Women’s Hormonal Supplements Market Size Worth US$ 8.50 Billion By 2032, Increasing Awareness of Women’s Health and Wellness Drives Growth | Research by SNS Insider.
- Verified Market Reports.








