μsmin® Plus is an innovative diosmin formulation (>80% pure diosmin and 90% total flavonoids) derived from hesperidin found in bitter orange (Citrus aurantium L) that demonstrates enhanced absorption and bioavailability compared to standard micronized diosmin.
In a double-blind, crossover study, µsmin® Plus showed greater plasma concentration and 9.4 times greater bioavailability compared to standard micronized diosmin.
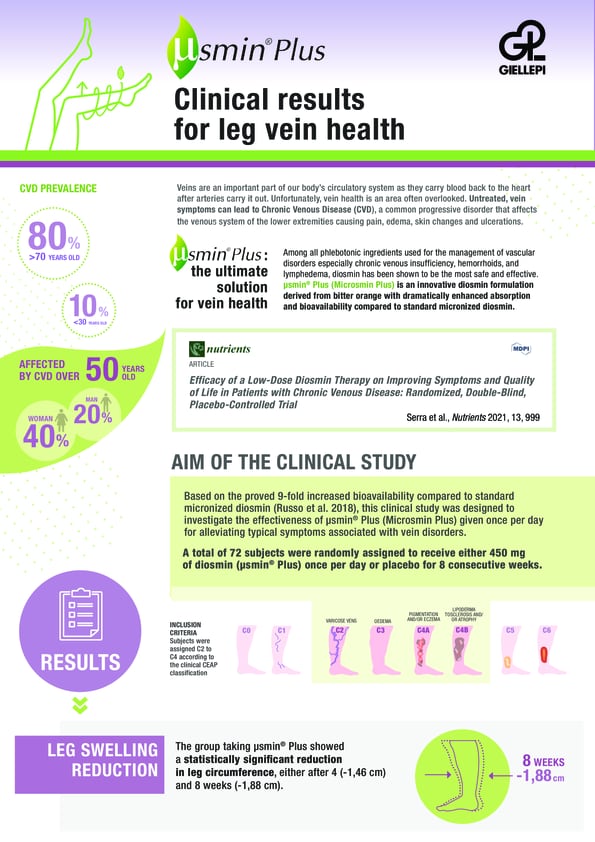
Today Giellepi is pleased to announce the publication of a new clinical study on µsmin® Plus (Microsmin Plus), titled “Efficacy of a Low-Dose Diosmin Therapy on Improving Symptoms and Quality of Life in Patients with Chronic Venous Disease: Randomized, Double-Blind, Placebo-Controlled Trial”. The results published in Nutrients further strengthen µsmin® Plus’ superiority in the diosmin marketplace showing dramatic improvements in leg circumference, quality of life (QOL), leg pain and common symptoms associated to Chronic Venous Disease (CVD).
In this eight-week study, subjects were randomized to receive either one tablet of µsmin® Plus (equal to 450 mg of diosmin) or a matched placebo tablet. Key outcomes included changes in mid-calf circumference and QOL measured by the Global Index Score (GIS). Also assessed was changes in pain, overall symptom severity as well as patient and investigator assessment of effectiveness.
Compared to the placebo group, the µsmin® Plus had a statistically significant reduction in leg circumference at both 4 and 8 weeks. At 8 weeks, QOL was significantly improved in the µsmin® Plus group compared to placebo. Overall pain relief was greater in the µsmin® Plus with 42% reduction reported at 8 weeks. Overall symptom relief was also more greatly reduced during the study.
Notable is the fact that investigators and subjects gave very favorable ratings to the reduction of symptoms in the µsmin® Plus group. The study found that 68% of the subjects in the µsmin® Plus group experienced a reduction in symptoms at two weeks compared to only 11% in the placebo group. When asked about overall satisfaction with the treatment, investigators responded that they considered µsmin® Plus to be good to excellent in 97% of subjects. In subjects, 100% responded that they were satisfied or very satisfied and 95% decided to continue using µsmin® Plus after the study finished.
“We are delighted with the results of this study which revealed the ability of µsmin® Plus in quickly delivering leg vein health benefits at almost less than half dosage recommended with conventional micronized diosmin”, says Dr. Rosario Russo Scientific Officer at Giellepi. “The highly bioavailable formulation of µsmin® Plus along with these new findings will help our customers further differentiate and standout in a marketplace dominated by micronized diosmin ingredients.”
μsmin® Plus is the only diosmin on the market providing up to 9X better absorption than standard micronized diosmin and proven efficacy at low-dose regimen.