The article 14 claim reads: “Lactobacillus fermentum CECT5716 decreases the Staphylococcus load in breast milk. High Staphylococcus load in breast milk is a risk factor for mammary bacterial dysbiosis/mastitis.”
In January this year Biosearch Life was granted a European patent for the use of “mammalian milk microorganisms, compositions containing them and their use for the treatment of mastitis”.
“Currently mastitis is treated with analgesics but this treatment does not solve the infection problem,” its scientific director Dr Mónica Olivares said at the time.
“In cases of mastitis showing severe pain, antibiotics are prescribed. However, the microorganisms responsible for mastitis usually generate biofilms and antibiotic resistance which causes failed therapies or recurrences that ultimately lead to the abandonment of breastfeeding.”
One in ten
Mastitis is often caused by a build-up of milk within the breast because of feeding problems.
In some cases this built-up milk can also become infected with bacteria such as Staphylococcus.
According to a review of data by the World Health Organisation (WHO) in 2000, the incidence of mastitis and breast abscess occur in all populations regardless of breastfeeding norms, with reported rates varying from few to 33% of lactating women.
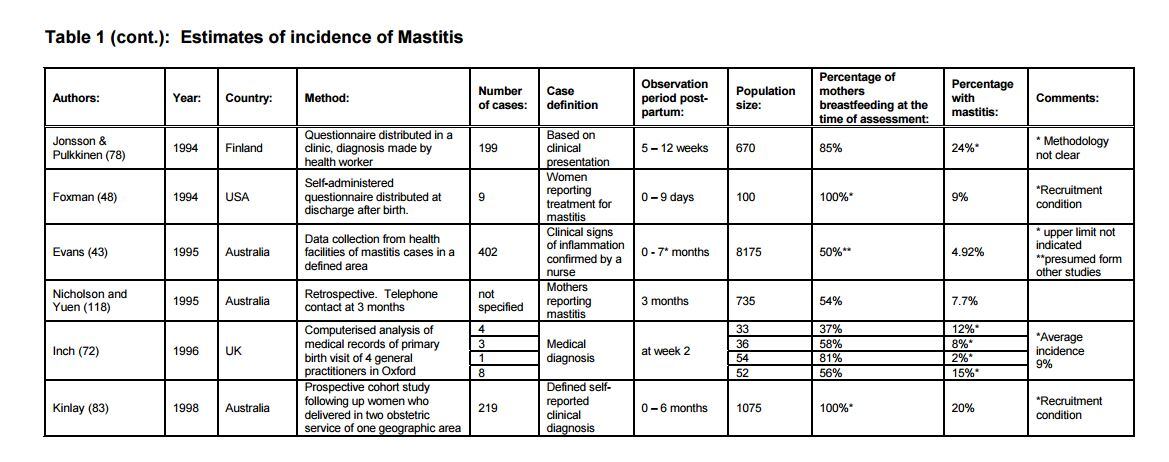
The UK’s National Health Service (NHS) estimates up to one in every ten breastfeeding women are affected by mastitis, usually within the first three months after giving birth.
The condition causes the breast tissue to become painful and inflamed.
It can also lead to breast abscesses if not treated and it is estimated that one in ten women who develop abscesses are unable to breastfeed using the affected breast again.
Health claim pending
According to the European Food Safety Authority’s (EFSA) register of questions the health claim will be processed within the legal deadline for such dossiers, meaning the Parma-based authority has five months to complete its evaluation once the application is considered complete.
Article 14 claims under the European nutrition and health claims regulation (NHCR) refer to children's development or health or to the reduction of disease risk.
EFSA’s dietetic products, nutrition and allergies (NDA) panel has so far received 268 applications, 103 applications of which were withdrawn.
To date 75 article 14 scientific opinions have been adopted.
