The variety of multivitamin-mineral (MVM) supplements in the marketplace gives consumers significant choice and an opportunity to find formulations with additional ingredients that may be of interest. It also gives companies the opportunity to differentiate their products in a crowded segment.
But clearly a B-vitamin complex, for example, is quite different from a Centrum Silver, but both could be classed as multivitamins.
While talk of a definition may be a redundant issue for many companies and consumers, it does present challenges when MVMs are considered in the context of federal or international aid programs.
Tensions
Dr Duffy MacKay, ND, senior vice president of scientific and regulatory affairs for Council for Responsible Nutrition, told us that there is always going to be tension between nutrition moving towards a personalized approach and the need or desire for a standard definition.
“Nutrition is not a one-size-fits-all approach,” he said, “and we see that in the market with multis targeting different age groups and genders. But it’s fair to say that for nutrition-based policies you need to be working with a defined product.”
There is no official definition of MVMs, but the US National Institutes of Health defines MVMs as: “[A]ny supplement containing 3 or more vitamins and minerals but no herbs, hormones, or drugs, with each component at a dose less than the Tolerable Upper Intake Level (UL) determined by the Food and Nutrition Board—the maximum daily intake likely to pose no risk for adverse health effects.”
NIH further classifies MVMs into three subgroups:
(1) “once daily,” which contain most or all vitamins and essential minerals at levels approximating the Recommended Dietary Allowance (RDA) or Adequate Intake (AI);
(2) special formulations designed for specific subpopulations, and packs containing multiple individual supplements; and
(3) “specialized” formulations that might contain vitamins and minerals at levels substantially above the RDA and sometimes the UL.
SNAP & WIC

A definition was included in H.R. 3841, the SNAP Vitamin and Mineral Improvement Act of 2017 introduced by Rep Mike Roger (R-AL), to “amend the Food and Nutrition Act of 2008 to make certain multivitamin-mineral dietary supplements eligible for purchase with supplemental nutrition assistance program benefits”.
According this bill, a multivitamin and mineral supplement:
(1) provides at least half of the vitamins and minerals for which the National Academy of Medicine establishes dietary reference intakes, at 50% or more of the daily value for the intended life stage per daily serving as determined by the Food and Drug Administration; and
(2) does not exceed the tolerable upper intake levels for those nutrients for which an established tolerable upper intake level is determined by the National Academy of Medicine.
Dr Daniel Fabricant, PhD, CEO of the Natural Products Association, is not optimistic that this will pass into law, and thinks there is a better chance for MVMs under the WIC (women, infants, and children) program administered by the USDA. Virginia Representative Dave Brat introduced H.R. 3529 last year to allow for multivitamin dietary supplements to be included in WIC food package.
UNIMAP
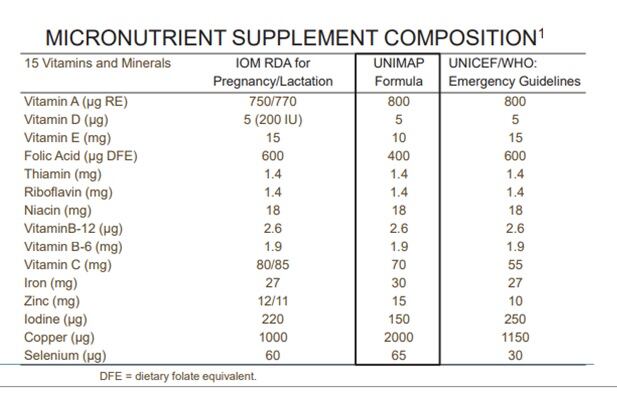
While there may not be a standard definition – and we could not find a single country that has attempted this – there are standardized formulations. In the context of international aid programs, a supplement called UNIMAP – or the UN Multiple Micronutrient Preparation – was developed in 1998 by UNICEF as a prenatal micronutrient supplement. The supplement is formulated with 15 vitamins and minerals at approximately the RDAs for pregnancy (see figure below).
Show this formulation to many supplement brands and it’s likely they will tell you they could improve it (with choline and other nutrients and bioactives), but the formula has been vetted and approved by international experts, said CRN’s Dr MacKay.
“A meta-analysis of 12 trials that evaluated the efficacy of the UNICEF 'UNIMAP' multiple micronutrient supplement […] concluded that several outcomes were significantly better than the comparison intervention, which was usually iron [plus] folic acid; birth weight increased by 22 g, low birth weight decreased by 11%, and small-for-gestational-age deliveries decreased by 10%,” stated Lindsay Allen, from the USDA, Agricultural Research Service Western Human Nutrition Research Center, University of California, Davis, Davis in a paper in Advances in Nutrition (2014; Volume 5, Issue 3, Pages 344S–351S).
“In the policy arena, these kinds of established formulas will get more traction,” said Dr MacKay. “In the business arena, we’re moving towards taking cheek swabs and then recommending multivitamins just for you.”
Multivitamin-mineral meta-analyses

The issue of a definition does come in to play when scientists pool results from intervention or observational trials into a meta-analysis. In many cases, these analyses pool together data from multiple studies, many of which do not employ the same intervention.
For example, a 2013 meta-analysis published in the American Journal of Clinical Nutrition (Macpherson et al. Vol. 97, pp. 437–444) examining the potential link between MVMs and mortality defined the products as, “a supplement containing [at least] 3 micronutrients, except when supplements contained [at least] 3 B vitamins only.”
In comparison, a meta-analysis published in the British Medical Journal (2005, Vol. 330, pp. 871) examining the potential role of MVMs in preventing infections in elderly people just considered studies that labeled their interventions as “multivitamin-mineral supplements”. The trials included in this study used supplements that contained between five and 25 vitamins and minerals.
“For meta-analyses you have to ask the right questions and weight it appropriately,” said NPA’s Dr Fabricant, and pointed to the meta-analysis methodology his association did in response to FDA’s proposal to revoke a law authorizing use of heart health claims for soy protein.
Opportunity
Prof Jeffrey Blumberg, PhD, senior scientist at the Jean Mayer USDA Human Nutrition Research Center on Aging at Tufts University, is lead author on a new paper from an international team of experts, published this month in Clinical Therapeutics (Vol 40, pp. 640-657). The experts used a modified Delphi process to reach a consensus on the role of MVMs in supporting human health.
“For the purpose of broad-spectrum micronutrient supplementation for a general population, MVMS should contain at least the micronutrients that are commonly under-consumed relative to their recommended intakes within that country/region,” the wrote. “Most of these vitamins and nutritionally essential minerals should be present in amounts approximating recommended intakes. Within this context, MVMS may be safely formulated for large subgroups according to age, sex, and/or life-cycle–specific micronutrient needs.”
This may sound vague, but it needs to be because, from an international perspective, each country will have different requirements. A multivitamin should be formulated differently in Indonesia or Eastern Europe, Prof Blumberg told us.
“Regardless of whether you’re eating an American diet or an Asian diet, some nutrients are going to fall short,” he said. “Not a lot of food contains vitamin D, for example. The classical effort has been to fortify foods – milk with vitamin A and D, salt with iodine, refined flour with folic acid. This is a public health recognition that there are shortfalls.”
It is these shortfalls that provide a real and rational and important need for a multivitamin-type product, he said.
The Clinical Therapeutics paper goes on to discuss the potential benefits of these supplements: “Use of MVMS is one approach to ensure that adequate micronutrient needs are met in support of biological functions necessary to maintain health,” they wrote. “Long-term use of MVMS not exceeding the upper limit of recommended intakes has been determined to be safe in healthy adults.”
While there is insufficient evidence to support a role for MVMs to prevent or reduce the risk of chronic disease, “for certain otherwise healthy subpopulations (eg, pregnant women, older adults) and some individuals with existing medical conditions who experience inadequacies in micronutrient intake, addressing inadequacies by using MVMS can provide health benefits.”
CRN’s Dr MacKay told us he’s surprised the dietary supplements industry hasn’t looked more to formulating products that address nutrient shortfalls.
“I often think there would be value in an organization like CRN to put out a best practice formula or benchmark for products for men, women, teens and so on,” he said.

