What is the HoA?
The Heads of Food Safety Agencies (HoA) represents governmental bodies in Europe responsible for food safety risk management.
The HoA promotes collaboration between competent authorities of participating countries in order to verify compliance with food law legislation, exchange views and experiences which will be beneficial for the improvement of efficiency and effectiveness and improve harmonisation of the enforcement within Europe.
The HoA meets biannually, usually in the country that holds the Presidency of the European Council. These plenary meetings aim at encouraging mutual cooperation and exchanging experiences and views on good risk management practices within. The HoA initiate Working Groups to develop and approve of “tools” and “best practices” which benefits the collaboration between the agencies and their outcome.
Representatives of the Directorate-General for Health and Food Safety of the European Commission (SANTE) and the European Food Safety Authority (EFSA) regularly take part in the meetings. They contribute the EU’s position and gain applied knowledge for their work.
The HoA working group on Food Supplements (HoA WG FS), comprising 26 member states, was established to create a common approach for managing and assessing certain substances used in food supplements
Recently the HoA group Food Supplements published a Report with a list of substances that should not be used or should only be used with restrictions in food (food supplements) due to their potential risk to human health (2024).
Summary of the HoA report
The report from the Heads of Agencies (HoA) working group on Food Supplements addresses the regulation and assessment of substances with nutritional or physiological effects in food supplements across the EU.
First of all, it is very important to highlight that this report is not part of the EU decision making process that foresees the active involvement of the European Commission that decides whether the legal conditions are met to launch Art. 8 procedure and provides EFSA with a mandate to undertake a safety assessment of the concerned substance. Therefore, before taking any action the European Commission will have to carefully analyse the Report.
Here is a summary of the key points highlighted in the HoA report:
Overview
- The use of various substances in food supplements is not fully harmonized within the EU, leading to inconsistent regulations and potential risks to consumers.
Objectives and Actions
- The working group aimed to compile a list of substances that should be prohibited or restricted in food supplements due to potential health risks.
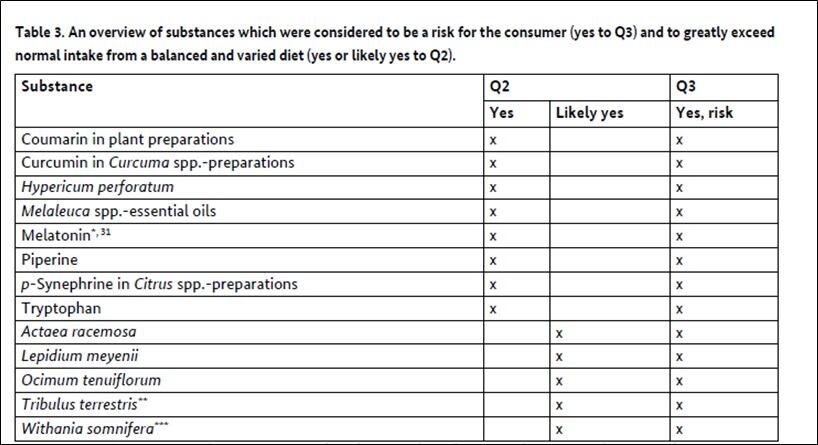
- A total of 117 substances were reviewed, focusing on their safety, novel food status, and potential for exceeding normal dietary intake when consumed through supplements.
- 13 substances were identified as high priority for regulatory action due to significant risks, including melatonin, piperine, and curcumin in Curcuma spp.-preparations.
Recommendations
- The report recommends that these substances undergo the ‘Article 8 procedure’ under Regulation (EC) No 1925/2006 to establish legal status and restrictions in EU food law.
- Substances deemed novel or with insufficient data should be grouped by priority and reviewed further to support EU-wide regulatory decisions.
Challenges and Next Steps
- The lack of a uniform risk management approach across member states complicates enforcement and consumer protection.
- The report suggests continued collaboration among EU member states and the EU Commission to refine the list of regulated substances and implement harmonized measures.
EHPM concerns
First of all, The European Federation of Health Product Manufacturers EHPM emphasizes that Food supplements are subject to a set of strict EU regulations in the framework of the European Food Law that ensure a high level of safety and quality and, most importantly, consumers protection (see detailed paragraph below).
EHPM believes that in order to properly address safety concerns regarding food supplements industry should gather data to support the safety of the products. Existing national notification systems and nutrivigilance systems are valuable tools to further guarantee the safety of food supplements marked in the EU.
The EHPM supports its members by providing tools and resources to further enhance safety and quality, advocating for compliance to regulations and adherence to best practices, and representing industry interests in regulatory discussions.
Specifically on the Report, EHPM is concerned about:
1) A potential misleading use of Art. 8 of Reg. 1925/2006 procedure
HoA WG intends to use Article 8 of Regulation (EC) No 1925/2006 for the substances listed in the report.
Article 8 of Regulation (EC) No 1925/2006 needs to be applied when a safety risk arises and not for an arbitrary classification purpose. EHPM is concerned about the fact that the HoA’s approach may lead to a misleading use of the Art .8 procedure for classification purposes, which could set a concerning legal precedent.
What is Article 8 of Regulation (EC) No 1925/2006?
Article 8 allows the European Commission to initiate – on its own or at the request of a Member State – the prohibition, restriction or to put under Union scrutiny a substance other than vitamins or minerals or an ingredient containing such a substance that is added to foods or used in the manufacture of foods.
2) Overstepping of Food Safety Agencies’ Remit:
This approach, in fact risks to overstep the remit of food safety agencies and encroach upon risk management responsibilities reserved for the European Commission and Member States
Food safety agencies are tasked with conducting risk assessments, not risk management. The latter falls under the remit of the European Commission after a risk assessment is conducted by EFSA or Member States Safety Agencies.
EHPM Concern: If food safety agencies are directly influencing risk management decisions, it could undermine the regulatory process and encroach upon the responsibilities of the appropriate authorities.
EHPM highlights the need for clear separation between risk assessment (by EFSA and National Food Safety agencies) and risk management (by EU bodies and Member States).
3) Disproportionate Approach Based on Hazard Rather Than Proven Risk:
EHPM is also concerned about the methodology advocated in the HoA report. In fact, banning substances based on potential hazards, rather than proven risks is not compliant to the regulation and would end up in a disproportionate risk assessment and management measures.
- There is a critical difference between ‘hazard’ (potential for harm) and ‘risk’ (likelihood of harm occurring). Banning substances based solely on their hazard profile without clear evidence of actual risk (i.e., exposure levels that lead to harm) is disproportionate and could unjustly impact the industry.
- EHPM advocates for a risk-based approach rather than a hazard-based one. This means substances should only be restricted if there is clear evidence of risk under realistic conditions of use, supported by robust data.
4) Gathering Safety Data and Post-Market Surveillance:
As detailed in the EHPM Guideline on post Market surveillance EHPM will mobilize its members to collect and provide safety data, including real-world post-market surveillance data, will be critical in demonstrating the actual safety profile of substances in question.
EHPM Encourages its members to compile comprehensive safety dossiers, including any adverse event reporting and exposure data, to provide a factual basis for discussions on safety and regulatory decisions.
Food supplements are high quality, safe and well-regulated products and EHPM is providing tools to its members to enhance safety and quality
Food supplements are governed by a comprehensive regulatory framework that ensures their safety and quality. Food supplements in the European market must comply with stringent quality and safety standards set by various regulations, including the EU Food Supplements Directive (2002/46/EC) and the General Food Law Regulation (EC) No 178/2002. These regulations ensure that supplements are safe for consumption and meet specific quality criteria. Manufacturers are required to use ingredients that are safe and appropriately labeled, and they must adhere to good manufacturing practices (GMP) to maintain product integrity and quality.
The regulatory framework for food supplements in the EU is designed to ensure that products are effective, safe, and accurately labeled. This includes rigorous pre-market assessments, adherence to established safety limits, and ongoing post-market surveillance to monitor product safety. Regulations also mandate that claims made about supplements are supported by scientific evidence, ensuring that consumers are not misled about the benefits of these products.
EHPM plays a crucial role by providing its members with the tools and resources necessary to maintain high standards and enhance consumer trust in these products. EHPM supports its members by providing resources and tools that help enhance the safety and quality of food supplements such as: the 3rd edition of the EHPM Quality Guide [more info here], the EHPM guidelines for food supplement companies on the management of adverse event reports [more info here], and the EHPM Probiotics Guidelines & Position Paper [more info here].
EHPM has also developed solid proposals to contribute to the resolution of long-standing challenges for the food supplements regulatory framework, such as: the EHPM proposal for “Maximum and minimum levels for vitamins and minerals: food supplements for adults and children sold in Europe” [more info here], and the EHPM proposal for a graded approach for “Botanical Health Claims on Foods & Food Supplements in the EU” [more info here].
Our aim is to ensure that food supplement regulations remain fair, evidence-based, and appropriately balanced between safety and consumer choice.

