The European Food Safety Authority (EFSA) is being heavily criticised, with food makers saying its rules make it harder to compete with other parts of the world.
But food and beverage manufacturers, along with ingredients suppliers, say they have a solution.
The problem with EFSA’s food safety assessment process
EFSA’s role is to evaluate the safety of food and drink across the bloc. This includes ‘novel foods’, which are foods not regularly consumed in Europe prior to 15 May 1997, the date the Novel Foods regulation was established.
With the European Commission currently evaluating EFSA’s performance over the last eight years, industry is taking the opportunity to share its impression of the food agency’s effectiveness in recent times.
The general feeling is that EFSA takes too long to assess food products and ingredients, discouraging business from entering the EU market. Efficient risk assessment processes are “crucial” to encouraging the adoption of innovation, believes food manufacturing trade body FoodDrinkEurope, among others.
The novel foods approval system in particular has a reputation for being slow. As a result, many companies prefer to submit applications in other markets before approaching the EU.
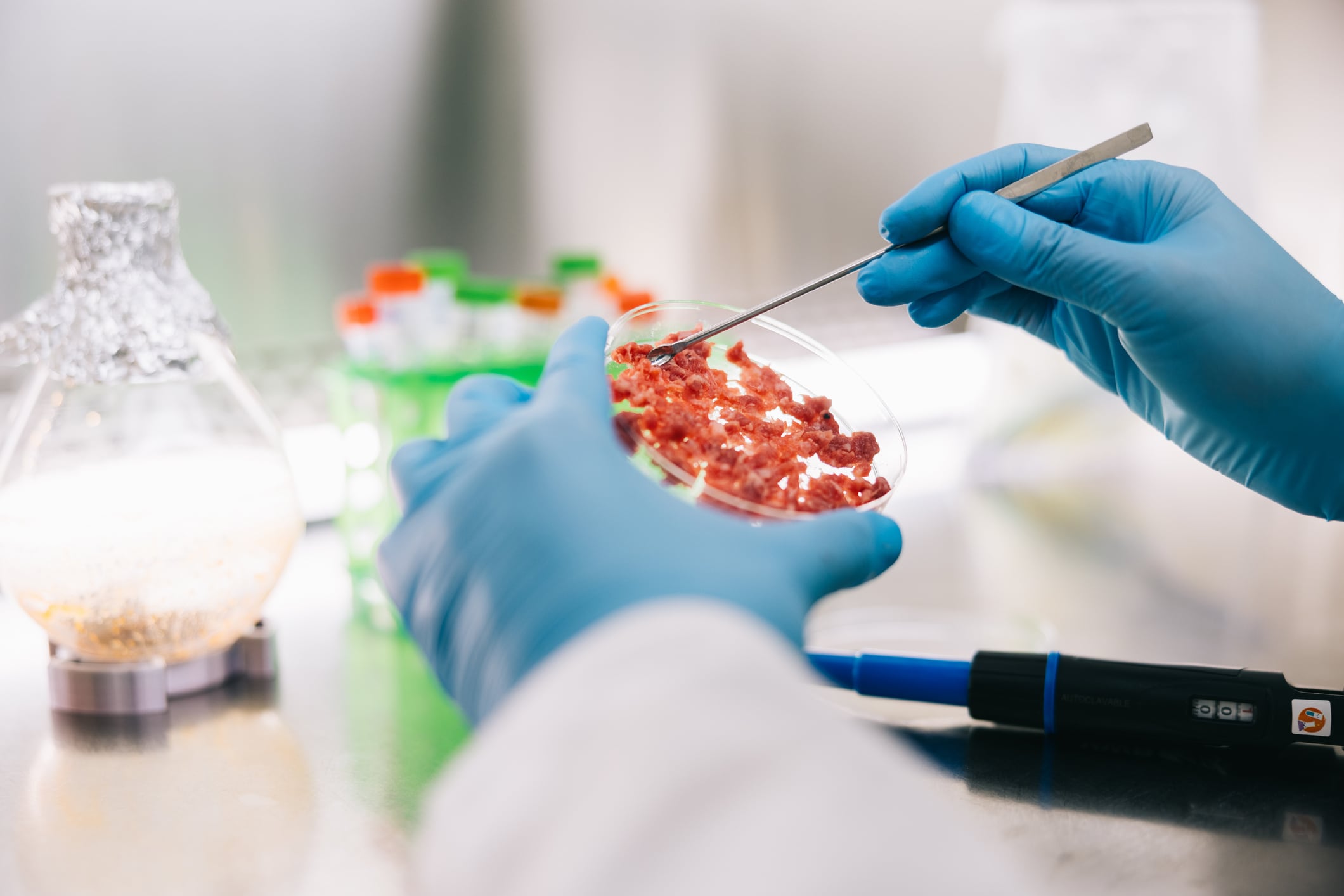
Take cell-cultivated meat, for example, which has seen submissions (and some approvals) in Singapore, the US, Israel, Switzerland, and the UK. The first approval was granted in Singapore five years ago. No cultivated meat product has been approved in the EU, and just two companies have applied.
Submitting a novel food ingredient application to EFSA has become “overly complex”, according to EU Specialty Food Ingredients (EUFSI), which understands the initial review phase alone now takes around seven months instead of 30 days.
EUFSI and FoodDrinkEurope are among those calling for the Commission to “speed up” EFSA’s performance evaluation, and want their frustrations to be heard.
It’s expensive to submit food safety applications to EFSA
One of the main concerns from industry is that it’s costly to submit food safety applications to Europe’s food agency.
The process places “significantly increased” administrative burden on companies, according to industry, which also incurs costs when hiring extra employees to manage the application process. More specialised training for these staff members is another financial burden.
Further, with projected timelines being lengthy, it’s felt that businesses often prioritise other geographies outside of the EU. “This shift also influences decisions on maintaining R&D facilities in the EU, given that innovative products tend to follow different market pathways.”
The solution, outlined by EUFSI, FDE and others, is to shape EFSA’s Transparency Regulation (TR) to help Europe stay competitive, support innovation, and reduce reliance on other regions.
What is the EU Transparency Regulation?
The Transparency Regulation (TR) aims to improve transparency and sustainability of food risk assessment, including for novel foods.
The TR, which came into force in 2021, brought several changes to the risk assessment process:
- Citizens can access scientific studies and information submitted to EFSA;
- Public consultations are now part of the authorisation process for regulated products;
- EFSA is informed of all studies relevant to authorisation applications, and the Commission can request EFSA to commission further studies if needed.
Reducing the ‘administrative burden’ of food safety assessments
Another concern expressed by industry lies in the administrative burden associated with food safety assessments in Europe. Streamlining administrative procedures and prioritising “core” scientific work could help ease the load, industry believes.
This administrative burden is not helped by the General Pre-Submission Advice (GPSA) and Renewal Pre-Submission Advice (RPSA), which companies say often repeat information they can already find online. A better approach might be to offer more pre-submission advice (like the European Medicines Agency does in life sciences), and increase interactions between EFSA and applicants during the risk assessment, say stakeholders.
Regulatory submission processes are also getting more complex, leading to more clarification questions and longer evaluation timelines. A new guidance document is required, and industry needs to be involved in its development, they say.
Another factor delaying the process is linked to an increase in requests for additional data, which industry doesn’t always believe is necessary. Data requests should be limited to what is “essential” to the safety assessment, and backed by clear scientific reasoning.
Criticism from industry expected by EFSA
The Commission’s evaluation of EFSA’s performance is due by March 2026. Although no one outside of the Commission knows yet how it will prevail, the food safety agency is optimistic.
“I’m convinced it will be a good outcome‚” EFSA executive director Bernhard Url told us last month. “There will be the desire from the industry for EFSA to be faster and more reactive. I won’t be surprised if this is the outcome, and maybe calls for more applicant support.”
Both these points have since been made by industry stakeholders.