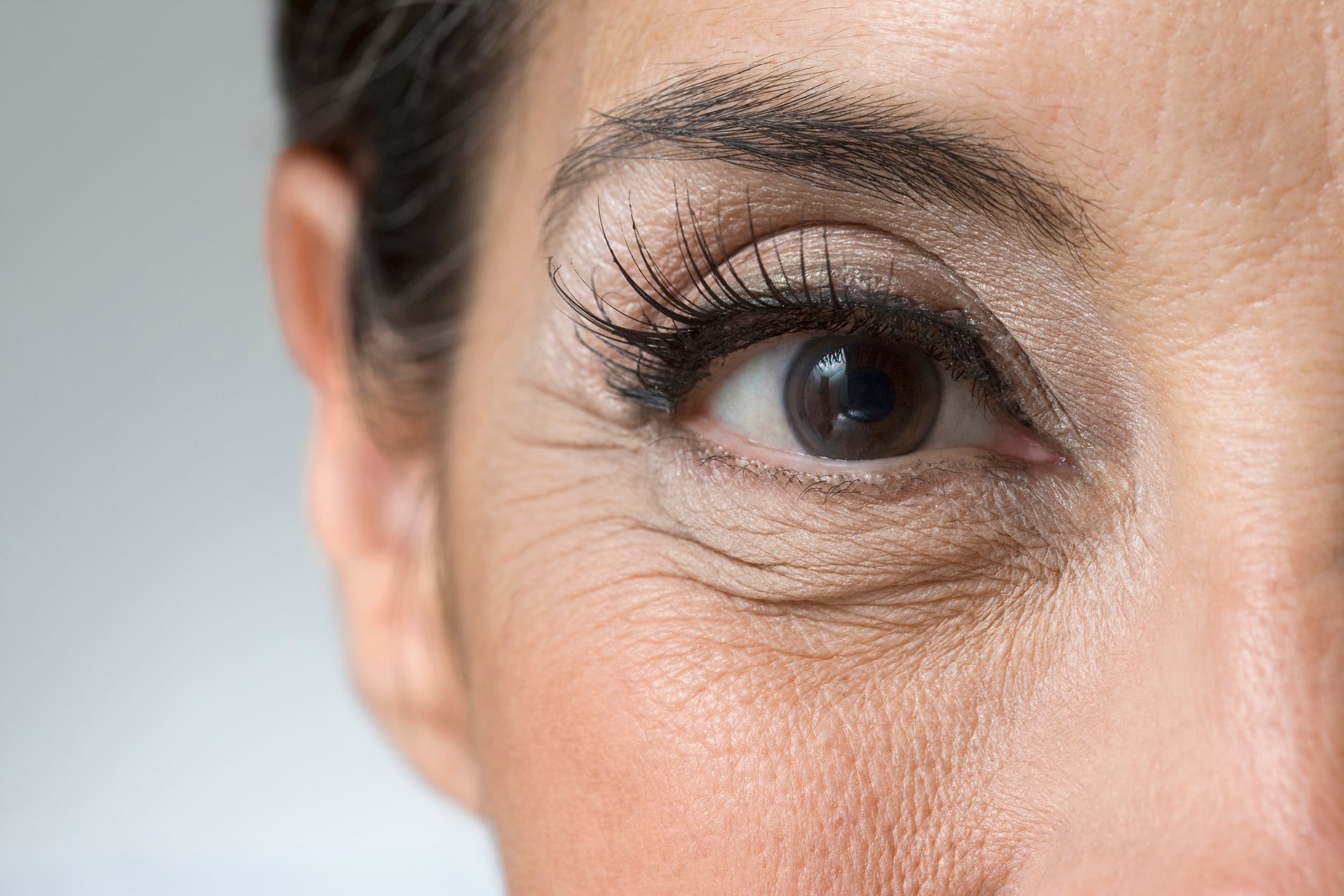
The skin improvements such as under-eye wrinkle appearance, skin hydration and barrier function were particularly evident among older postmenopausal women identified as S-equol producers.
The declines in estrogen at menopause can lead to accelerated skin aging. Some women may address this with hormone replacement therapy (HRT), but not all women may be suited to the medication due to side effects or contraindications.
Plant-derived compounds such as isoflavones may mimic estrogenic activity. Gut microbiota convert the isoflavone compound daidzein into the metabolite S-equol. S-equol is considered to be the most biologically active metabolite due to a greater infinity for estrogen receptors.
“However, not everyone possesses the necessary gut bacteria to produce S-equol. Some estimates suggest that in East Asian populations, 50% to 80% of individuals are S-equol producers, whereas this rate is only 20% to 35% in Western populations—a discrepancy largely attributed to the lower dietary exposure to soy,” the researchers from ADM noted.
They highlighted that the benefits of soy isoflavones to menopausal symptoms and bone health may be largely dependent on an individual’s ability to produce S-equol.
“Therefore, S-equol should be considered when evaluating the impact of isoflavone supplements on skin health as well,” they wrote.
Commenting on the findings, Dr. Richard Day, vice president, medical affairs and clinical development at ADM, told NutraIngredients that while group level differences were “modest,” they observed that women who produced higher levels of S-equol showed improvements in skin parameters.
“Response appeared to be greater among S-equol producers, consistent with the biology of the gut–skin axis and S-equol’s estrogen-like activity,” he said.
“For the skincare and healthy aging market, these findings support a personalized, microbiome-aware view of beauty-from-within. Isoflavone supplementation may particularly benefit skin appearance and resilience in individuals capable of producing S-equol.”
Novasoy Isoflavone supplement linked to higher S-equol levels
The researchers randomly assigned 66 healthy postmenopausal women between the ages of 40 and 60 to consume either 200 mg of Novasoy, providing 80 mg of soy isoflavones in glycoside form, or a placebo for 12 weeks.
Skin assessments were made at the start, midpoint and end of the study using calibrated equipment, and urine samples were analyzed for soy isoflavones. The researchers also used a validated soy food-frequency questionnaire at the beginning of the study to estimate soy consumption.
The results revealed a 5.62% reduction in the Novasoy group for the average crow’s feet roughness compared to a 1.65% increase in average wrinkle roughness in the placebo group. However, the difference did not reach statistical significance, and there was no significant difference in other skin parameters, such as under-eye wrinkles, hydration or skin color.
There was a significant difference in urinary soy metabolites such as genistein and daidzein between the groups, and “S-equol levels were also 1.56 times higher in the Novasoy 400 group than in equol producers in the placebo group,” the researchers wrote, noting that higher S-equol levels were significantly associated with improved under-eye wrinkles and transepidermal water loss at the study midpoint.
“Moving forward, skin health remains a key focus area for ADM’s research, across both human and companion-animal health,” Dr. Day told NutraIngredients.
Source: Frontiers in Nutrition. doi: 10.3389/fnut.2025.1671835. “S-equol status modulates skin response to soy isoflavones in postmenopausal women: results from a randomized placebo-controlled pilot trial”. Authors: V. Vijayakumar et al.