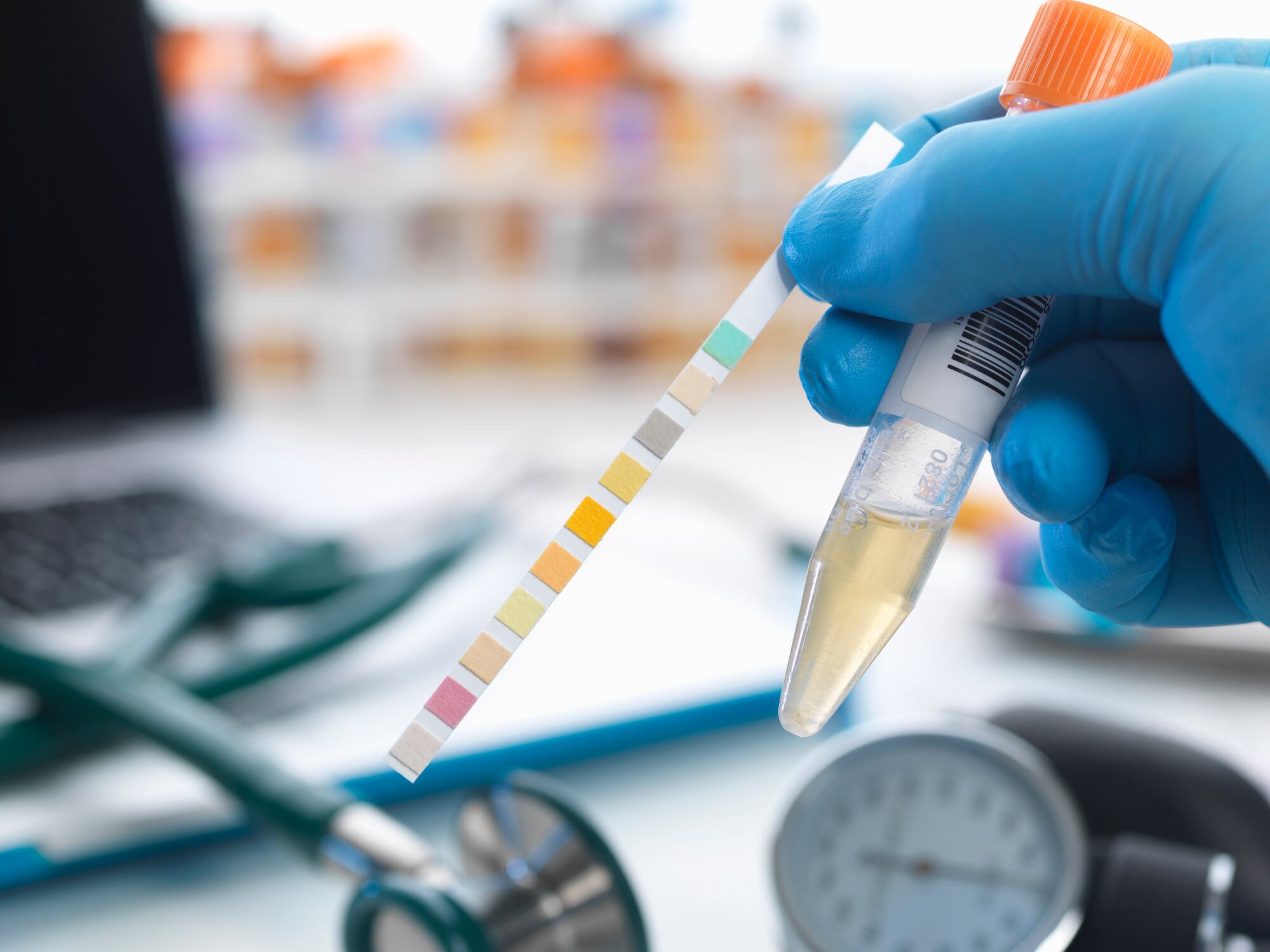
The new test, which is slated to debut in Q3 2026, will measure immunoglobulin A (lgA) levels—an indicator of plasmacytoid dendritic cells (pDC) activity—in urine samples. A ‘commander-in-chief’ of the immune system, pDCs activate immune cells such as NK cells, T cells and B cells.
Previous research from Kirin reported that individuals with high pDC activity have significantly higher levels of lgA in their urine samples. The findings are planned for publication in Q2 this year.
Visualizing immune status to promote immune care
Kirin said that the urine test could help individuals “visualize” and better understand their immune status.
According to Kirin, there is an awareness-behavior gap when it comes to immune care in Japan.
A survey on 10,000 Japanese adults between the ages of 20 and 69 reported that eight in 10 (85.4%) agreed that immune care is needed to stay healthy.
However, when asked the priority level of immune care among overall health practices, only about 11% have immune care habits, such as consuming foods, beverages or supplements for immunity daily.
Therefore, the company believes that introducing a visualization tool can make immune status more visible and give individuals reasons to take action for their immune health.
“We believe that understanding one’s immune condition can serve as an important trigger for interest in and engagement with immune care,” a spokesperson from Kirin Holdings Company Limited, Research & Development Division, Institute of Health Science, told NutraIngredients. “Through the insights gained from the testing service and educational initiatives on immune care—including improvements in daily habits—we aim to broaden public understanding and involvement in immunity."
The urine test that it is piloting provides information on a person’s lgA levels at the time of testing, trends over time and recommends steps to improve immune status.
“The urine test for IgA—an indicator linked to pDC activity that we plan to introduce—is intended to ‘visualize’ one aspect of innate immunity that fluctuates with lifestyle and other factors.”
Trial on LC-plasma in Australia, Malaysia
Kirin is also conducting clinical trials in Australia with Blackmores and Griffith University on its flagship immune health ingredient Lactococcus lactis strain Plasma postbiotic, commercialized as LC-plasma or IMMUSE.
The aim is to formulate a new health supplement and apply for a health claim with the local Australian authority using findings from the trial.
“The product scheduled for launch will be a health supplement developed by Blackmores, and the specific formulation and product specifications are currently under consideration,” the company spokesperson said. “Since overseas markets have health‑claim regulations that differ from those in Japan, we are exploring the possibility of applying different types of claims by utilizing the scientific findings that will be obtained from this clinical study."
The trial with Blackmores involves 600 adults between the ages of 18 and 60, who will consume either a 50 mg daily dose of LC-plasma or a placebo for 24 weeks. It will assess the effects of LC-plasma on upper respiratory symptoms, including the duration, occurrence and severity.
The trial with Griffith University involves 220 adults between the ages 18 and 60, who will consume either a 50 mg daily dose of LC-plasma or a placebo for four weeks. Here, the participants’ upper respiratory symptoms, immune markers including pDC activity and antigen detection of influenza A/B, RSV and coronavirus will be assessed. The testing of pDC activity will also be broadly assessed by Cytometry by Time-Of-Flight (CyTOF) to identify novel markers of pDC activity.
Elsewhere in Southeast Asia, Kirin is partnering with infectious disease expert Professor Dr. Sazaly Abu Bakar to study the potential of LC-plasma against dengue fever.
Professor Sazaly is also the founder and executive director of Tropical Infectious Diseases Research and Education Centre at the Universiti Malaya in Kuala Lumpur.