
US imposes antidumping and countervailing duties on HECs from Brazil, China, India and Vietnam
A recent ruling could have dramatic implications on pricing, supply chains and the overall competitive landscape of Hard Empty Capsules.

A recent ruling could have dramatic implications on pricing, supply chains and the overall competitive landscape of Hard Empty Capsules.

FDA eases ‘no artificial colors’ claims, but ambiguity over ‘petroleum-based dyes’ leaves manufacturers cautious

A Republican congressman has introduced legislation seeking to reaffirm federal oversight of dietary supplements, blocking states from imposing their own additional regulatory requirements and creating what he characterized as a “confusing patchwork of...

Mouth pouches have emerged as one of the fastest growing CPG categories, signaling a major trend in nootropics as consumers seek functional ways to boost performance—sans nicotine.
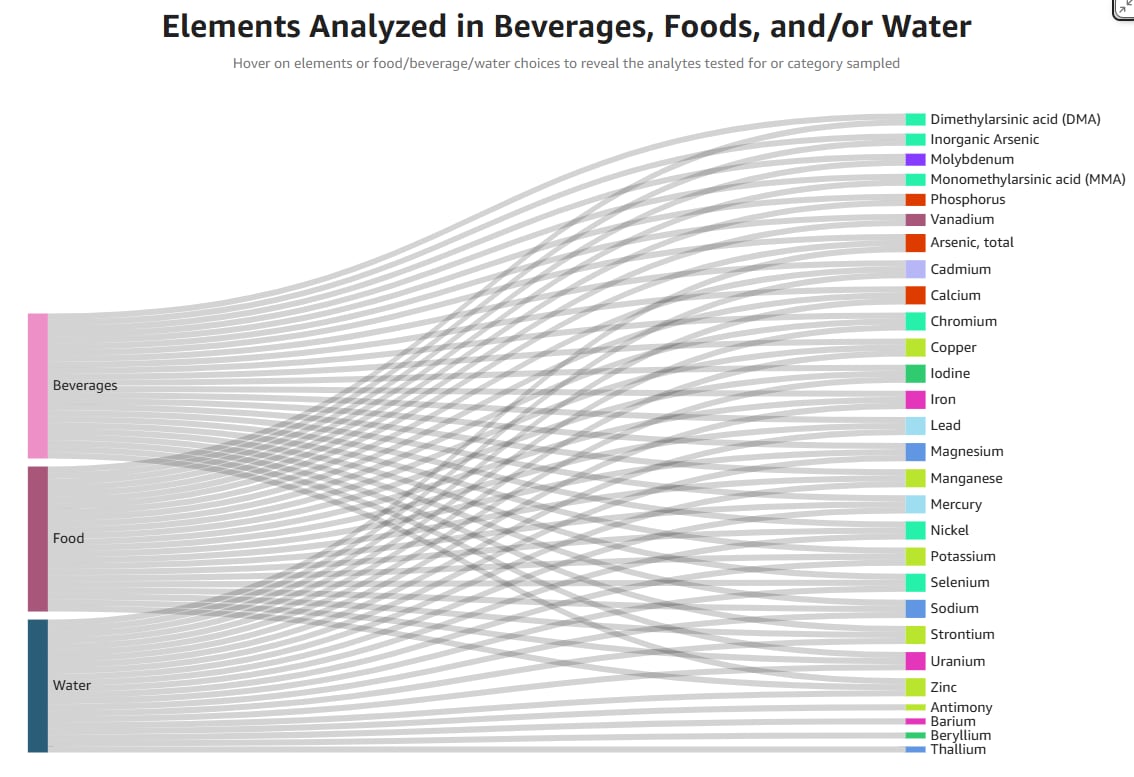
The FDA’s new Total Diet Study Interface is already being used to challenge state-level contamination claims, offering a clearer view of nutrient and contaminant data in the US food supply


Lawmakers in Hawaii have filed a bill to prohibit the sale of weight loss and muscle building products to minors, the latest in a long list of state-level attempts to restrict access to specific dietary supplement categories.

A bipartisan bill in the U.S. House of Representatives introduced on Jan. 22 could establish the first comprehensive federal regulatory framework for hemp-derived products intended for human consumption.

Catch up with our weekly round-up of key news from across the Nutraverse.

The NutraIngredients Sports and Active Nutrition Summit (SANS) 2026 in San Diego, CA, is less than a month away! Day Two’s lineup will be focused on regulatory preparedness, emerging cellular health science, and evolving opportunities in healthy aging...

Following the release of the Human Food Program 2026 Deliverables last week, the Food and Drug Administration’s Office of Dietary Supplement Programs (ODSP) is communicating its priorities for dietary supplement oversight in the year to come.

Guest article
In a recent column, Daniel Fabricant of the Natural Products Association argues that mandatory product listing (MPL) is anti-MAHA, anti-innovation and a threat to the regulatory balance established by DSHEA. But that argument rests on a false premise:...

The U.S. Food and Drug Administration Human Foods Program’s 2026 priority deliverables, published on Friday, are drawing mixed reactions from dietary supplement industry stakeholders, many of whom expressed concern over innovation constraints.

Catch up with our weekly round-up of key news from across the Nutraverse.

The Functional Mushroom Council (FMC) has launched as a nonprofit industry organization representing North American functional mushroom growers, suppliers and brands, with a focus on research, education and quality standards.

The Alliance for Natural Health USA (ANH-USA) has filed a federal lawsuit against the U.S. Food and Drug Administration (FDA) over the agency’s recent rejection of a petition to allow over 110 ‘government-backed’ disease risk reduction health claims.

Scott Bass is launching a new firm—Scott Bass Life Sciences LLC—focused on two core offerings: high level strategic guidance for the global self-care sector and a low-cost dispute resolution model designed specifically for life sciences companies.

Guest article
The Make America Healthy Again (MAHA) movement is built on a simple but powerful promise. Our government should expand access to preventive and wellness tools and reject one-size-fits-all bureaucratic solutions that treat citizens as incapable of making...

U.S. Senate Democratic Whip Richard Durbin (D-IL) has introduced the Dietary Supplement Listing Act of 2026, which would require dietary supplement manufacturers to list their products with the Food and Drug Administration (FDA).

Delivery formats are becoming a growing compliance risk under DSHEA, warns industry attorney Jennifer Adams.

A lawmaker in Alaska has pre-filed a bill to prohibit the sale of weight loss and muscle building products to minors.



During a press conference Wednesday, Health Secretary Robert F. Kennedy Jr. announced new dietary guidelines that flip the traditional food pyramid on its head.

The U.S. Food and Drug Administration (FDA) has denied a petition from the Alliance for Natural Health USA (ANH-USA) seeking to allow over 115 ‘government-backed’ disease risk reduction health claims.

Do you have an initiative, program or project that produced significant improvements for the wider industry? If yes, NutraIngredients USA wants to hear from you for its Editors’ Award for Industry Initiative of the Year.

Tech giant Amazon is rolling out its requirements for all dietary supplement sellers on the platform to use a third-party testing, inspection, and certification (TIC) organization to demonstrate cGMP compliance, according to the American Herbal Products...

Banned Substances Control Group’s Oliver Catlin recently partnered with the Associated Press to expose something shocking: research peptides marketed as “laboratory chemicals” are flooding mainstream platforms like Amazon and Alibaba.

Guest article
For nine decades, the Natural Products Association has occupied a singular role within the dietary supplement industry: When its businesses are threatened, NPA fights.

Research shows that some fish oil supplements are oxidized, but is there any scientific evidence to support the perception that oxidized fish oil is less efficacious or even detrimental?

The Trump Administration issued an executive order today directing federal agencies to finalize the rescheduling of marijuana to Schedule III and to develop a new regulatory framework for hemp-derived cannabinoid products, including CBD.

Guest article
As the American Herbal Products Association (AHPA) readies for the new year, we pause to reflect on a period of profound change and robust growth, navigating an increasingly complex landscape of regulation, market trends and policy shifts. This year, we...

We enjoy writing some articles more than others, whether it’s because of the subject, the impact for industry, the insights from commentators or other reasons. But which articles did we enjoy writing the most and why?

Turmeric- or curcumin-containing natural health products (NHPs) in Canada must update their labels to warn about the risk of hepatotoxicity, following a review by Health Canada of the available information.


In a letter to the dietary supplements industry, the FDA’s deputy commissioner for human foods says the agency is considering a regulatory amendment regarding requirements around the DSHEA disclaimer on product labels.

Guest article
Transparency has suddenly become one of the most talked-about themes in today’s food and consumer safety debates. Policymakers are pressing for more visibility into the ingredients entering the food supply, and the momentum behind reforming the...

The IPA World Congress + Probiota 2026 is just two months away! The leading event for the probiotics, prebiotics and microbiome community is a must-attend event, and let us tell you why…

The U.S. Food and Drug Administration has sent letters to specific NMN ingredient suppliers confirming it has set aside previous determinations that the vitamin B3 form is excluded from the definition of a dietary supplement.

Guest article
What a year of transition, uncertainty and unpredictability 2025 has been—not just for the dietary supplement industry, but for all of us navigating rapid change. From shifting tariff rates to upheaval at FDA, from renewed pushes to restrict access to...

The National Institutes of Health has launched a five-year project to develop a unified model of healthy human physiology, signaling a shift toward whole-person health that aligns with longstanding approaches in the nutrition and supplements sector.

Tariffs meant to boost U.S. manufacturing might actually be pushing companies away from American investment. Chase Johnston, vice president of operations at Arjuna Natural, breaks down how rapidly shifting tariff rates are creating chaos for the...
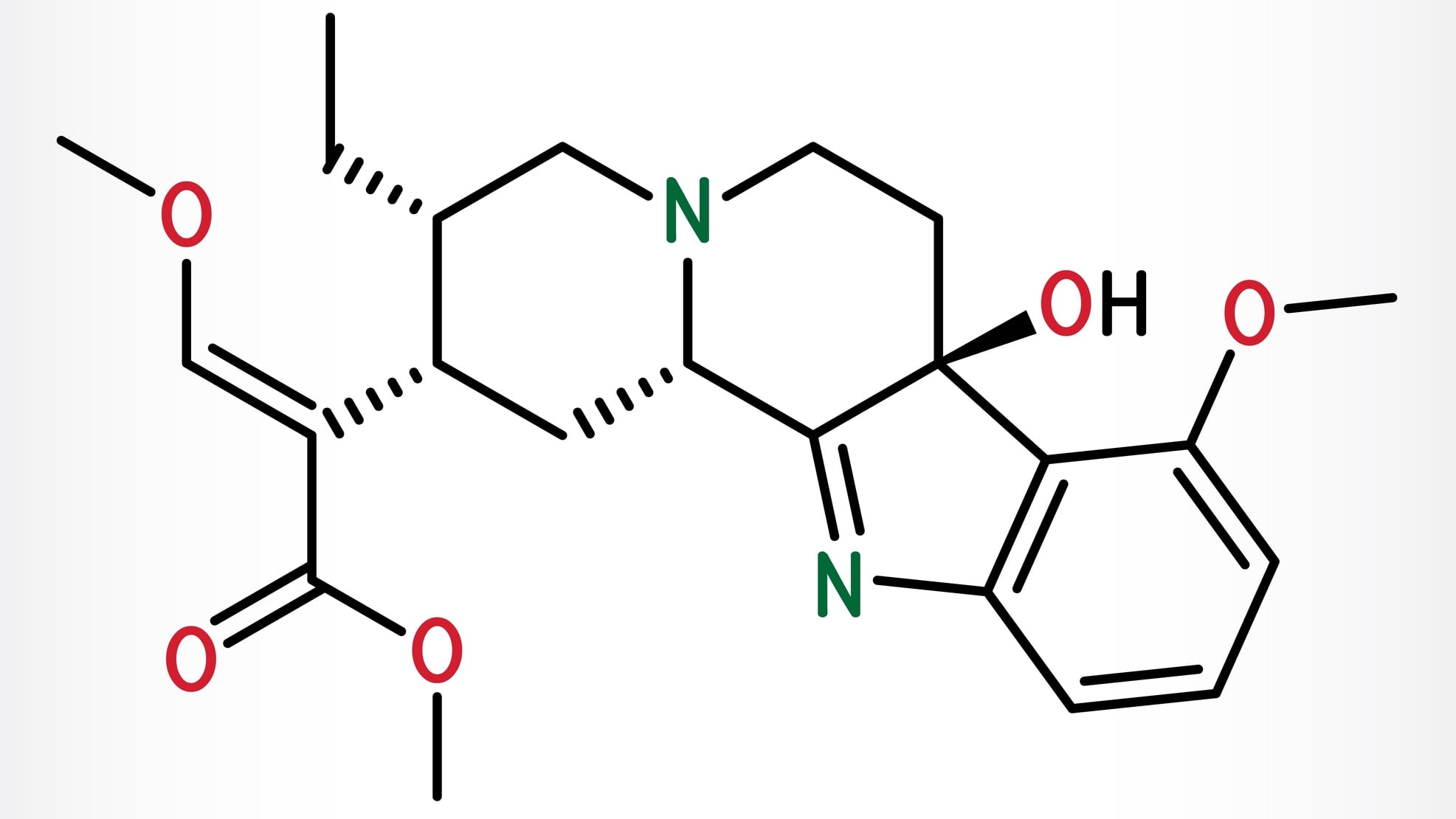
The U.S. Marshals Service has seized approximately 73,000 units of 7-hydroxymitragynine (7-OH) products from three firms in Missouri, the U.S. FDA announced.

Catch up with our weekly round-up of key news from across the Nutraverse.

The Council for Responsible Nutrition (CRN) has determined a Highest Observed Intake (HOI) level for vitamin K2-MK7 of 375 mcg/day following a review of over 40 clinical trials.


Apex Compliance’s Asa Waldstein shares valuable insights for those seeking to avoid an FDA warning letter, a competitor case with the NAD or a referral to the FTC due to ad claims non-compliance.

News in brief
The Department of Health and Human Services (HHS) has rescinded the reduction in force notices for staff involved with the National Health and Nutrition Examination Survey (NHANES) following public pressure to recognize the importance of the survey for...

A lawmaker in Michigan has filed a bill to prohibit the sale of weight loss and muscle building products to minors. The Council for Responsible Nutrition said the bill is “premised on a misunderstanding of both the science and the regulation of dietary...

The Trump Administration has revised and expanded the tariff exemption list to include botanicals such as green and black tea, cinnamon, ginger and turmeric.