In a statement released yesterday, Sanofi will add to its early-stage pipeline ORI-001, a candidate for acne vulgaris that is based on recombinant proteins and which entered preliminary clinical studies in Q3 2021.
Austria-based Origimm Biotechnology’s lead program is an immune therapy against Propionibacterium (Cutibacterium) acnes, the bacterium thought to cause the strong inflammatory response seen in the skin condition.
The program looks to support the human immune system in controlling P. acnes growth on the skin and prevent damage to the cellular lining of the pores and avoid any potential skin scarring.
Unlocking full potential
“The acquisition of Origimm further broadens our vaccines R&D pipeline with a first vaccine candidate against acne, a high medical need for millions of teenagers and adults,” says Thomas Triomphe, Executive Vice President, Global Head of Sanofi Pasteur.
“Welcoming Origimm within Sanofi expands our area of expertise by bringing extensive know-how in the field of skin microbiome and skin immunology. We look forward to unlocking the full potential of this candidate.”
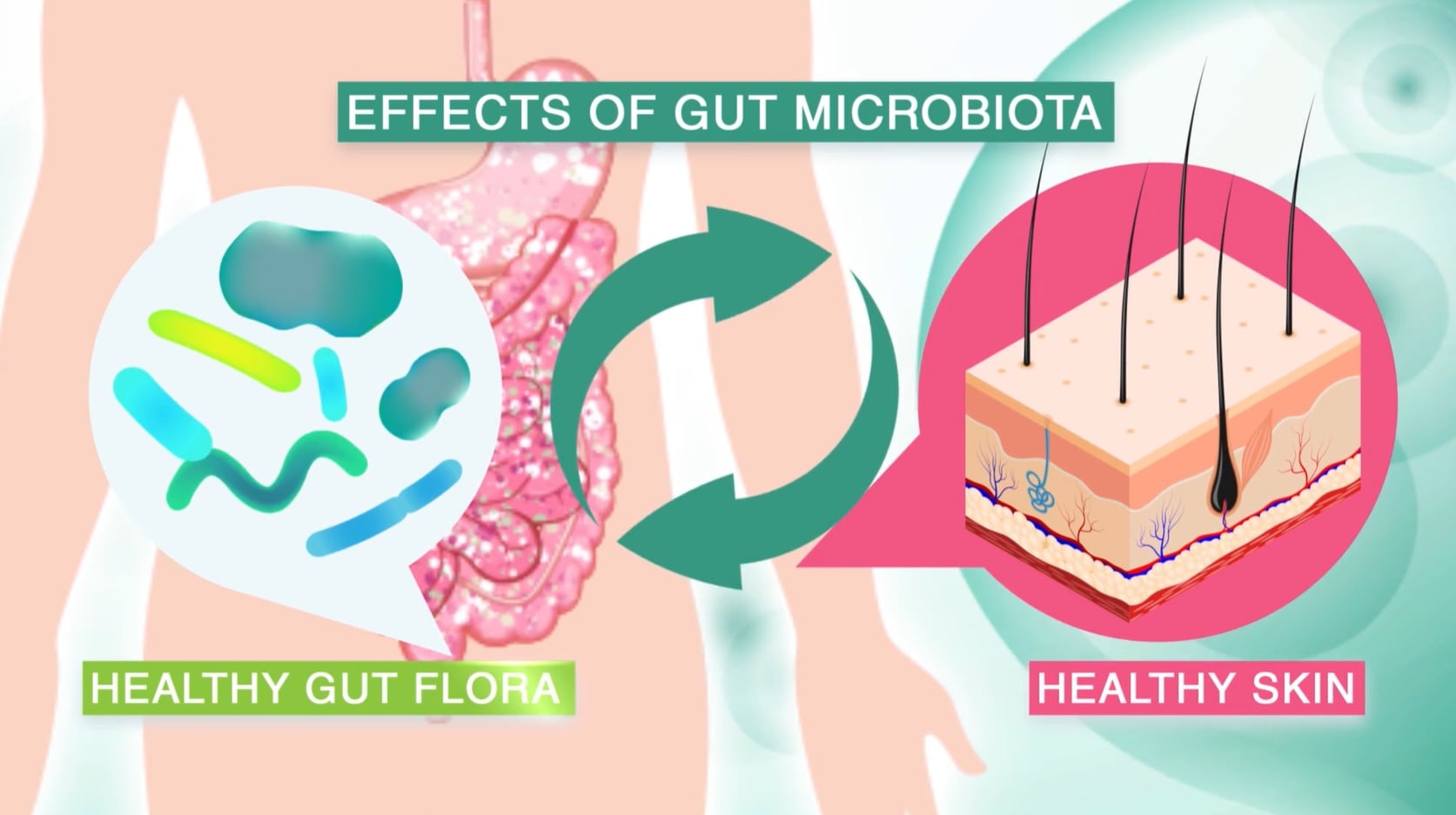
The agreement is the latest in a spate of skin health-related deals in which pharmaceutical firms have taken an interest in microbiome engineering possibilities to address disorders such as acne and eczema.
Fellow pharma rivals GlaxoSmithKline recently inked a €185m ($224m) deal with Eligo Bioscience to target the microbiome to reduce the risk of developing acne.
The agreement, finalised in January 2021, sees Eligo develop EB005, the Paris-based firm’s discovery program for acne.
If successful, GSK would then form a license and collaboration deal to further develop EB005, where Eligo could receive up to €185m in license fees, milestone payments as well as royalties on global sales.
Bayer and Azitra
Not to be outdone, German pharma firm Bayer entered a joint development agreement with Azitra that uses the skin microbiome as a basis for new skin care products that address a range of skin conditions.
The 2020 partnership focuses on Azitra’s Staphylococcus epidermidis strains and their potential as a main bioactive component in a new range of Bayer-developed skincare products under a future License Agreement.
Possible areas of application include medicated skin care products for sensitive, eczema-prone skin as well as therapeutic products for skin diseases such as atopic dermatitis.
Commenting on the acquisition that is expected to complete this month, Origimm’s Founder & CEO/CSO Sanya Selak, says, “We are looking forward to combining our expertise and strengths to continue developing innovative solutions for prevention and treatment of the skin microbiome-associated diseases, such as common acne.”
“Together with such a strong partner like Sanofi, we will strive to creating a paradigm shift in treatment of skin diseases and many other microbiome-associated disorders and infections, for which current medical solutions are inadequate.”