This evolving landscape was a central theme at the recent Nutra Healthspan Summit in London, where leading researchers and industry experts convened to critically assess the current state of geroscience and how it can be used to develop targeted interventions with age-defying effects.
According to some experts in the room, the science is struggling to keep up with the influx of new products that are spinning the hallmarks into cellular health claims on pack, encouraging consumers to take a leap of faith based on surrogate endpoints rather than hard outcomes.
“Cellular health is the microscopic version of overall health—the healthier our cells are, the healthier we are—sounds logical but you have to prove it,” said Carlos Unamunzaga, CEO of TetraSOD, which markets an ingredient derived from the microalgae Tetraselmis chuii.
T. chuii targets at least nine of the hallmarks of aging and has undergone in vitro and in vivo study. Research indicates that it stimulates antioxidant and anti-inflammatory mechanisms, primarily by activating transcription factor NRF2 and enzyme SIRT to enhance cellular defenses, supporting cognitive, metabolic and skeletal-muscle health.
Connecting hallmarks to outcomes
Commonly depicted by a wheel or circular diagram and first defined in 2013 by López-Otín et al. in a paper published in the journal Cell, the hallmarks of aging are presented as slices and layers in a network of bidirectional influence and causal cascade.
“Aging research has experienced an unprecedented advance over recent years, particularly with the discovery that the rate of aging is controlled, at least to some extent, by genetic pathways and biochemical processes conserved in evolution,” the paper noted at the time. “A major challenge is to dissect the interconnectedness between the candidate hallmarks and their relative contributions to aging, with the final goal of identifying pharmaceutical targets to improve human health during aging, with minimal side effects.”
López-Otín et al. initially identified nine hallmarks: genomic instability, telomere attrition, epigenetic alterations, loss of proteostasis, deregulated nutrient sensing, mitochondrial dysfunction, cellular senescence, stem cell exhaustion and altered intercellular communication. In 2023, the research team expanded the targets, adding disabled macroautophagy, chronic inflammation and dysbiosis to the list. Emerging hallmarks include altered mechanical properties and splicing dysregulation.
While these can be grouped into a hierarchical aging model of primary, antagonistic and integrative outcomes to trace the path towards mortality, how is industry connecting and translating this science—much of it still conducted in cell lines and animal and microscopic nematode models—into meaningful healthspan extension for consumers?
The hallmarks of aging
Primary Hallmarks (root causes)
1. Genomic instability: accumulation of DNA damage from endogenous and environmental sources.
2. Telomere attrition: progressive shortening of chromosome ends leading to replicative aging.
3. Epigenetic alterations: age-related drift in DNA methylation, histone marks and chromatin architecture.
4. Loss of proteostasis: misfolded, aggregated or insufficiently degraded proteins.
5. Mitochondrial dysfunction: impaired oxidative phosphorylation, reduced ATP, elevated ROS, altered mitochondrial dynamics.
6. Deregulated nutrient sensing: disrupted insulin/IGF-1, mTOR, AMPK and sirtuin pathways controlling growth and metabolism [sometimes included as antagonistic hallmark]
Antagonistic Hallmarks (responses that can become harmful)
7. Cellular senescence: permanent cell-cycle arrest with secretion of SASP inflammatory factors.
8. Disabled macroautophagy: reduced ability to recycle damaged organelles and proteins.
9. Altered intercellular communication: excessive inflammatory signaling, disrupted neurotransmission, impaired endocrine cues.
Integrative Hallmarks (final consequences)
10. Stem cell exhaustion: diminished self-renewal and regenerative capacity in tissues.
11. Chronic inflammation (“inflammaging”): persistent low-grade inflammation driving tissue dysfunction.
12. Microbiome disturbance (dysbiosis): age-related shifts in microbial composition disrupting immunity and metabolism.
13. Extracellular matrix (ECM) stiffness: increased cross-linking and structural changes impairing tissue elasticity and function.
14. Splicing dysregulation: impaired RNA processing leading to abnormal transcript variants.
15. Compromised adaptive stress responses: reduced ability to mount protective responses to metabolic, oxidative and proteotoxic stress.
Gabriele Civiletto, PhD, associate principal scientist at ingredient supplier dsm-firmenich, has focused his research on exploring the effects along cellular pathways to identify disease before symptoms manifest downstream. As not all the hallmarks are easily measured or actionable by dietary intervention, dsm-firmenich has zeroed in on cellular senescence, chronic inflammation, dysbiosis and mitochondrial function as more discernable marks.
“By targeting these outcomes of aging, designing precision interventions, we can decelerate the aging process,” Dr. Civiletto said during his presentation on nutritional strategies to slow biological age and support healthy lifespan extension. “But the situation is even more complicated because we know that these outcomes of aging interact with each other, and we are working to understand how targeting one outcome of aging also influences the others.”
On the market, dsm-firmenich is advancing its “Longevity Shift” initiative by proactively supporting healthy longevity through targeted nutritional blends. The company refers to the DO-HEALTH study, a three-year, randomized clinical trial that investigated effects of its vitamin D and omega-3 ingredients in combination with a simple home exercise program in close to 2,000 healthy seniors.
The DO-Health Research Group has produced several papers and post hoc analyses suggesting reduced odds of become pre-frail and exploratory findings of a cumulative reduction in cancer risk with the combination of the three interventions, as well as a 10% reduction in total falls and a modest slowdown of biological aging at the cellular level with omega-3 alone. No statistically significant benefits were found for blood pressure, physical performance, cognitive function, non-vertebral fractures and overall infection rates.

The beauty and skincare market is also embracing the healthspan-slash-longevity shift, with both L’Oréal Groupe and Estée Lauder launching longevity-focused products earlier this year that move beyond before-and-after claims to a proactive, pro-vitality approach that extends to skinspan.
During his presentation on targets for preserving cellular skin architecture, Rishabh Kala, PhD, director of advanced technology pioneering for skin biology at Estée Lauder, highlighted the company’s 17 years of research in longevity science. More recently, this research has evolved to focus on a group of seven signaling proteins, known as sirtuins 1 through 7, involved in metabolic regulation and cellular homeostasis.
“Why do we study sirtuins and skin? Skin is one of the largest organs,” Dr. Kala said. “It is the first barrier which protects us from all the exposures, […] and importantly, sirtuins are present in all skin layers or all skin cells, keratinocytes, fibroblasts and melanocytes.”
Through clinical study, the expression of these sirtuins is closely linked to the reduction of inflammation and production of essential compounds for youthful skin like collagen and elastin, translating to improvements in dark spots, lines and redness. Here, Dr. Kala explained, primary hallmarks are defined as root causes with downstream effects, such as DNA damage or telomere depletion leading to cellular senescence and, ultimately, inflammation.
After 15 years of research, L’Oréal Groupe introduced the Wheel of Longevity for Beauty “to decode skin aging at the cellular, molecular and tissular levels” based on nine hallmarks of aging using the company’s proprietary Longevity AI Cloud that analyzes over 260 skin longevity biomarkers to select the appropriate biological pathway for intervention.
“It is so complex that we had to design a tool to choose the biological pathways upon which we want to act, depending on the hallmark and the clinical sign that we want to target,” said Caroline Delaunay, global head of evaluation intelligence at L’Oréal, during a panel discussion on longevity in skincare. “What is really important is to really embrace the complexity of the mix between all the hallmarks and to act on which are the most important for each individual.”
L’Oréal also recently acquired a minority stake in Swiss longevity firm Timeline, which has also invested in 15 years of research, studying urolithin A—a natural metabolite of gut microbiomes with the bacteria capable of processing bioactive ellagitannins found in fruits, nuts and seeds—to support mitochondrial health by recycling and rejuvenating aging mitochondria.

Uniqueness, placebo and lowest-hanging fruit
One of the big questions across presentations was that of inter-individual response, reinforcing the idea that a one-size-fits all or a reductionist approach to the hallmarks and aging science may miss nuances that affect real outcomes.
Sean Gibbons, PhD, associate professor at the Institute for Systems Biology, reviewed how host physiology may influence aging dynamics in the context of the microbiome, where certain aspects of health in older adults can be reflected in how much their microbiomes change—or become more unique—with age.
“As we age, it seems like we’re drifting further and further apart from each other,” he said. “We’re becoming more and more unique—more unique snowflakes. This drift actually accelerates as we go forward through the next decades of life, and we’ve replicated this across several different cohorts.”
Whichever way the research team analyzed its clinical data, people who rated themselves as healthier showed a consistent pattern: As they aged, their microbiomes grew increasingly unique compared with others. In contrast, individuals in poorer health did not show this pattern. In fact, maintaining a more “youthful,” less distinctive microbiome into old age appeared to be the unhealthy signature, whereas ongoing change—reflected as rising uniqueness—was linked to better health and predictive of survival.
In addition, Dr. Gibbons discussed findings that not everyone responds the same way to interventions—in this case through the ability to produce beneficial butyrate, where uniqueness in older age was shown to be “very positively associated with predictive butyrate production.” He also noted during a subsequent panel discussion on mining the centenarian microbiome for lifespan insights that marrying measures to outcomes linked to frailty risk might be more moveable in the short term rather than tying them to the broader hallmarks of aging.
David Furman, PhD, director of the AI and Bioinformatics Platform at the Buck Institute for Research on Aging and head of the Stanford 1000 Immunomes Project, presented a multi-layered approach to understanding and measuring aging, focusing on inflammation, functional decline and organ-specific aging. He highlighted that while clinical trials show that targeted interventions can reduce inflammatory age, placebo effects are strong and difficult to beat in short-term studies unless exponentially increasing cohort size or lengthening study periods.
“So, both of those are really tough calls for companies—to increase sample size or duration,” Dr. Furman said, noting a third option. “You can also go to market, sell diagnostics and supplements and then use real-world evidence.”
In a field like aging research, where sufficiently powered human clinical trials should take decades and would require millions of dollars, citizen science and easy-to-track outcomes emerge as alternative to marketing derived from mechanistic plausibility and anecdotal results shared on social media.
Andrew Franklyn-Miller, chief medical officer and innovation at Nuritas, cautioned that solutions must not get ahead of themselves and over promise but that the field can approach certain segments like sleep, skin or muscle health that have broad-ranging implications.
“We know that in terms of muscle, in terms of longevity as a whole, muscle mass is actually the lowest hanging fruit because we have seen systematic review of meta-analysis data that up to 17% of all-cause mortality can be influenced by muscle improvements like grip strength or muscle mass,” he said.
The Irish biotech company uses artificial intelligence to discover and develop plant-based bioactive peptides including PeptiSleep to enhance sleep quality, PeptiYouth to reduce the appearance of wrinkles and PeptiStrong to improve muscle strength.
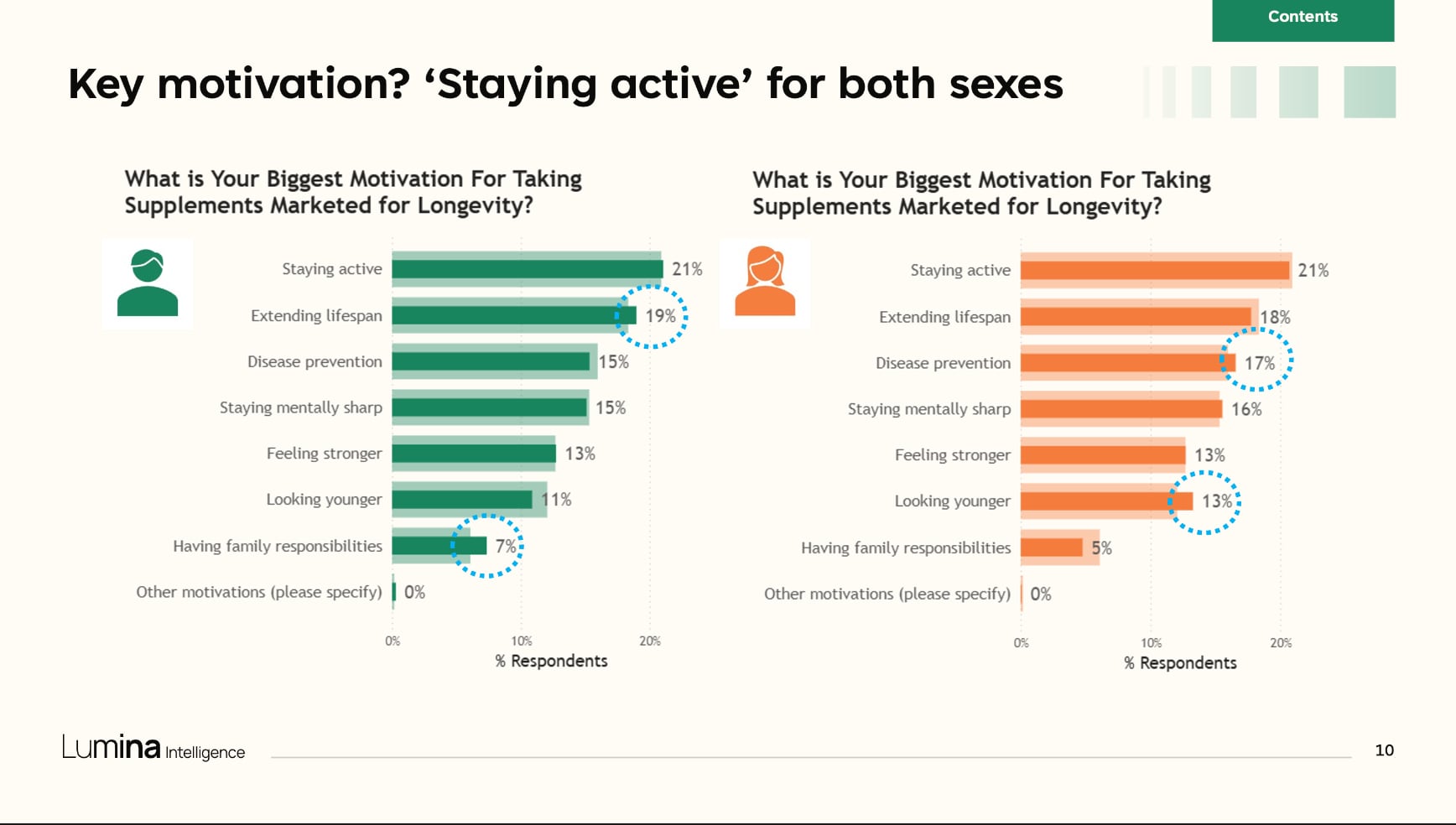
Data presented by Lumina Intelligence from its recent healthspan consumer survey identified sarcopenia, blood sugar control, metabolic health and cognitive decline among the top health concerns among a set of 3,500 respondents in the United Kingdom, United States and China. Among this cohort, staying active led as key motivator for taking supplements marketed for longevity, followed by extending lifespan, disease prevention, staying mentally sharp, feeling stronger, looking younger and having family responsibilities.
Commenting on endpoints, Linda Neckmar, senior vice president of human health at Novonesis, recalled the importance of remembering and continuing to educate about the purpose of dietary supplements.
“We will never be able to see anything like pharma drugs in the supplement space, so just having that expectation level set right—we are talking about healthy people trying to be healthier,” she said.
There are also the implications for trust and truth regarding how science and its unknowns are applied and communicated to consumers in a field where layered, multi-interpretable ideas cannot be reduced to one-dimensional content. For example, some summit speakers warned against intervening too aggressively or inadvertently on the hallmarks of aging, noting that while boosting telomerase might extend cellular lifespan, excessive activation might increase cancer risk for instance.
“When you simplify something, at some point, what you’re saying no longer becomes true, and that’s a really fine line to walk, is to convey the science and be in truth but also distill it down in a way that’s digestible,” said Shawn Wells, founder and CEO of ZoneHalo, who discussed how—as a formulator and science-driven biohacker—he balances risk and benefit for emerging ingredients.
Wells is, in scientific terms, an n-of-one experiment who reports a biological age of 33 at the age of 51 and has been self-testing longevity protocols for decades, well before correlation is validated. He has helped bring a variety of branded ingredients to market for energy, blood sugar regulation, muscle recovery, stress resilience and longevity through the nine-figure company he founded but also emphasizes the value of connection, curiosity, play, sleep and preserving the ability to adapt to hermetic stress as a foundation for building resilience.


