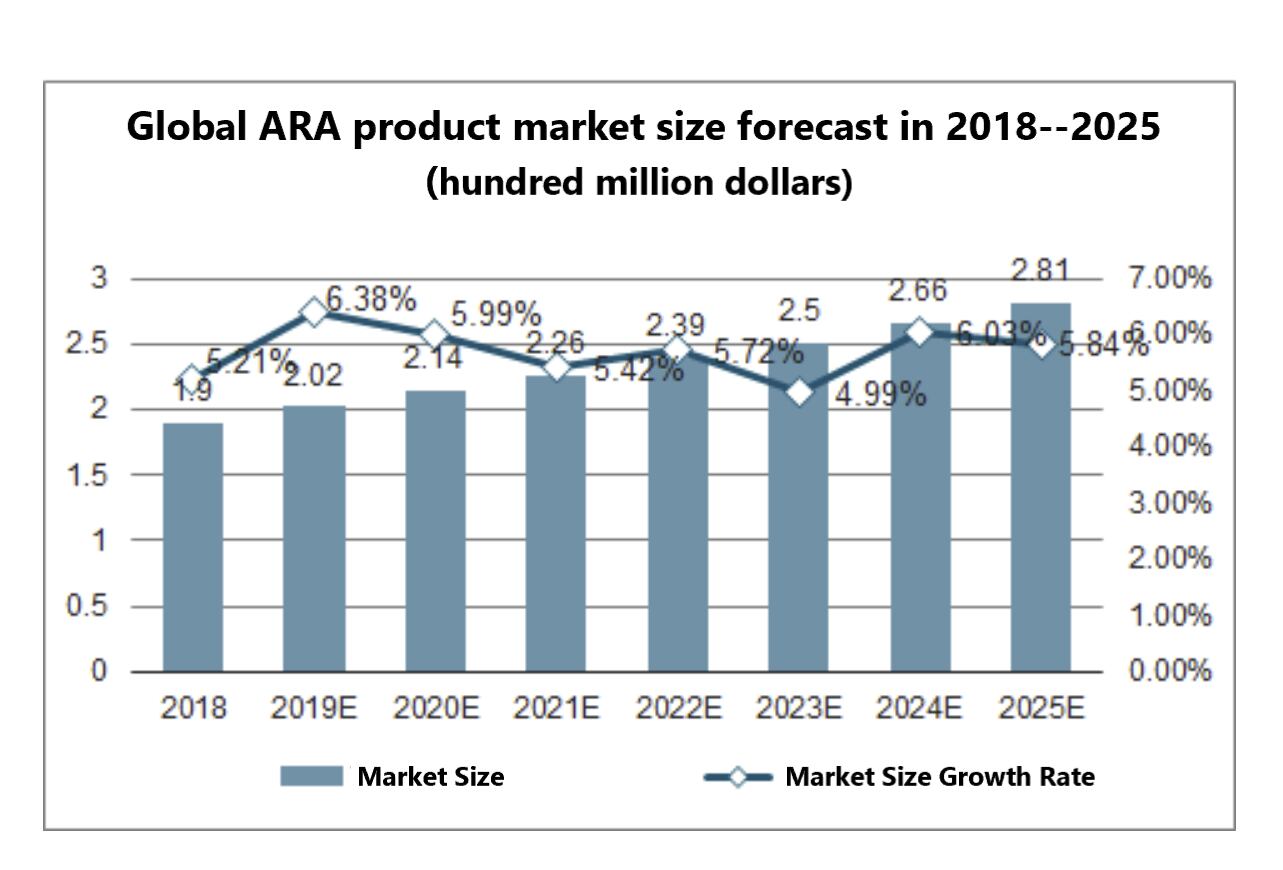
As the nutritional supplements market in high-income countries and groups gradually deepen and people pay more attention to their health, the effectiveness of dietary supplements has been constantly improved. Arachidonic acid, also known as ARA, is a kind of Omega-6 (or n-6) polyunsaturated fatty acid. Over the last decades, there has been an increased understanding of the molecular roles that the n-3 and n-6 PUFAs play in brain and cellular function. The variety of functions related to ARA indicates its importance and essentiality in the metabolic chain of events leading to brain structural lipid development, signaling, visual development, and other many essential cellular functions.[1]
Along with the deepening of research on ARA, ARA for the increased use as a food nutritional fortifier has become a trend, especially in infant formula, healthy food, and dietary supplements. In the current downstream field, infant food additives are the primary demand market of ARA, with a compound annual growth rate of 5%. Nutritional fortifier for health food is the second-largest application field of ARA products. Protein powder is one of the crucial sports nutrition products, and the market demand has continued to rise in recent years.
The primary sources of extraction of ARA are egg yolks and animal organs. As from 1995, ARA is also obtained through fermentation and produced on industrial scale.
Cabio’s patented strains and technology
The fungi Mortierella alpina is the only micro-organism approved by EFSA for industrial ARA production through microbial fermentation. With more than 20 years of technical development and financial input, Cabio has promoted the production and content of ARA in Mortierella alpina to industrial scale thus becoming the first supplier in China and the second supplier in the world to achieve mass production of ARA. 20 years ago, the Institute of Plasma Physics Chinese Academy of Sciences applied plasma technology, which using ultra-high voltage electricity to ionize inert nitrogen molecules into nitrogen ions, and forming rays to obtain mutant strain. In combination with a high-throughput system, strains with industrial production potential were screened by random plasma mutagenesis in tens of thousands of Mortierella alpina. These screened strain has the best performance characteristics such as efficiently convert glucose into long-chain fatty acid ARA in the process of fermentation, which is a milestone event of microbial synthesis of oil.
After intensive selection and screening for safety and best performance a specific MA strain has been selected and used by Cabio for the production of high quality ARA-oil. In 2016, this technology was awarded the Chinese national scientific and technological progress prize, which is a very prestigious award with the highest honor in the science and technology field. At present Cabio can guarantee a consistent high ARA-level in the oil.
3-MCPD and GE are challenges in oil production and processing
Microbial oils are prepared by microorganisms such as yeast, mold, bacteria, or algae under certain conditions, using carbohydrates, hydrocarbons as carbon and nitrogen sources. This method has many advantages, such as high oil content, short production cycle, not affected by the season, no occupation of farmland etc. However, during deodorization (which is essential to obtain the edible oil with good sensorial properties) processing contaminants like 3-MCPD and GE may be formed.
It is known that 3-monochloropropane-1,2-diol or 3-chloropropane-1,2-diol(3-MCPD) and gylcidyl esters (GE) are common contaminants in oil processing. GE is an esterification product of fatty acids and glycidyl, in oil deodorization, high temperature accelerates the decomposition of GE, which then combines with chlorine to form 3-MCPD. Studies have shown that 3-MCPD may also be formed in other processed foods, notably arrange of cereal products that have been subjected to heat treatments such as baking, roasting or toasting.
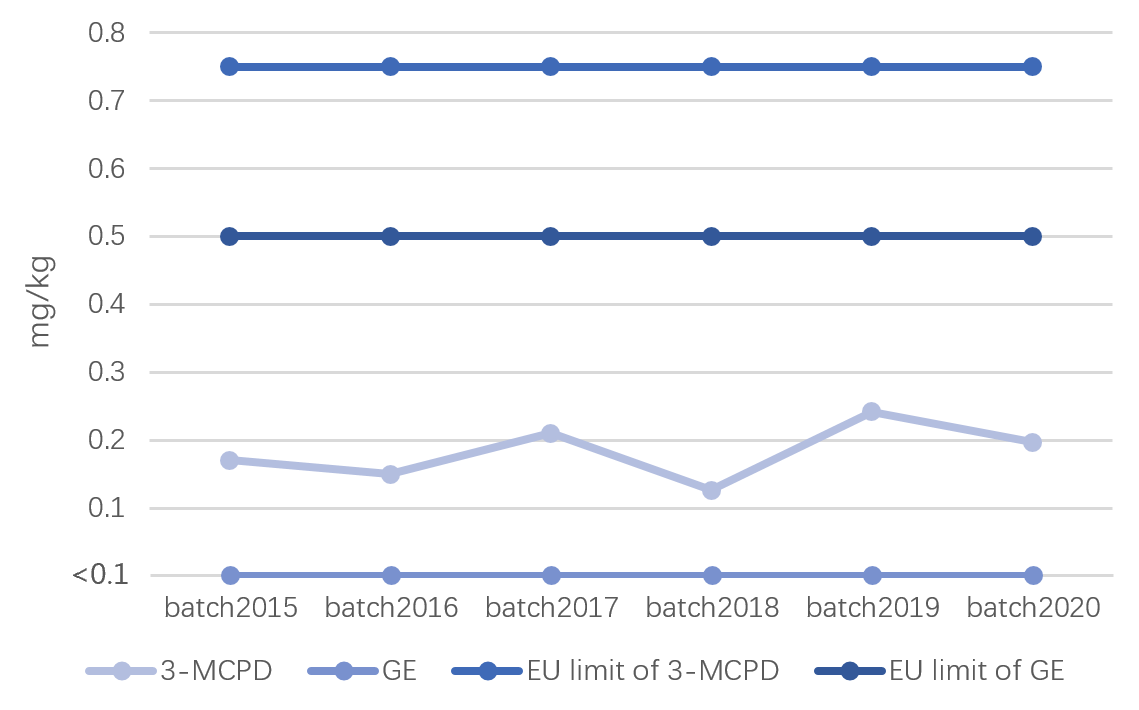
3-MCPD and GE as new food contaminants have attracted international attention in recent 10 years. The International Agency for Research on Cancer ((IARC) has classified 3-MCPD as "possibly carcinogenic to humans" (Group 2B), and GE as “probably carcinogenic to humans” (Group 2A) [2]. The European Food Safety Authority (EFSA) issued the limit standards for 3-MCPD and GE in food in 2020 [3], EU-legislation puts limits for 3-MCPD and GE in vegetable oils and fats, fish oils and oils from other marine organisms destined for the production of baby food. Max. 500 ppb for GE and 750ppb for 3-MCPD.
Cabio’s proprietary processes to control 3-MCPD and GE in ARA oil
Cabio continuously attached great importance to the monitoring of contaminants in its products, including 3-MCPD and GE. The contents of 3-MCPD and GE in ARA are controlled by Cabio’s patented technology [4-5], including lipase activity in microbial cells at the early stage of oil extraction, and fine control of adsorption process conditions to reduce the probability of catalytic synthesis of GE and effectively reduce the contents of 3-MCPD and GE in ARA. The levels of 3-MCPD and GE in ARA obtained are significantly lower than the limit requirements of the European Union.
CABIO is a biotechnology-based company which has been focusing on R&D, manufacturing and sale of polyunsaturated fatty acid ARA, algal DHA oil, Sialic acid, natural-sourced β-carotene and other microorganism-fermented nutrient products for 20 years.
With biotechnology as our pillar, CABIO will be offering global infant formula food and healthy food companies’ professional, safe and quality qualified nutrient products and solutions. At present, Cabio has already corporated with Cargill, Nestle ,Danone, abbott,Kerry, Givaudan,Feihe, Junlebao, Yili,, By-Health, H&H Group , Angel Yeast, etc. Through promoting public health via nutrition regulation, it aims at becoming a biotechnology company of advanced technology, global operation and sustainable development.
[1] Hadley K, Ryan A, Forsyth S, et al. The Essentiality of Arachidonic Acid in Infant Development [J]. Nutrients, 2016, 8(4): 216.
[3] Amending Regulation (EC) 1881/2006 as regards maximum levels of 3-monochloropropane diol (3-MCPD), 3-MCPD fatty acid esters and glycidyl fatty acid esters in certain foods
[4] Li X.Y., Lu S.H., Chen L, et al. Low-chloropropanol microbial oil and method for preparing same [P]. CN107418982 A, 2017-12-01.
[5] Wang Z.M., Chen L, Ma F.T., et al. A microbial oil and its preparation method [P]. CN107460035 A, 2017-12-12.






